How are the properties of the particles in a solid?
particles are held tightly in place, only vibrational motion
How are the properties of the particles in a solid?
particles are held tightly in place, only vibrational motion
How are the properties of the particles in a liquid?
particles slightly more spread out , able to slide past one another (freedom of movement)
How are the properties of the particles in a gas?
particles VERY spread out
List the 4 ideas of Kinetic Molecular Theory
- gas particles have insignificant volume
- particles are in rapid, random, constant motion
- collisions among particles are elastic
- Kinetic energy of the particle is proportional to the temp.
At what temperature do particles theoretically have no kinetic energy?
0 Kelvin or -273°C
Units for pressure
1 atm = 14.7 lb/in2 (psi) = 760 mmHg (torr) = 101.3 kPa
What is the relationship between the states of matter and their attractions?
Stronger attraction means more likely it will be a solid. Weaker attraction means more likely it will be a gas.
What is the relationship between particles, pressure, and collisions?
More particles, more collisions between the particles means more pressure
What is Vapor Pressure?
the pressure exerted by a gas vapor above it’s liquid surface a measure of how quickly/easily a liquid evaporates
What happens in order for Vapor pressure to increase?
Temperature increase
When do liquids boil?
When their Vapor pressure equals the external pressure (usually atmospheric pressure)
What are Crystalline solids?
Solids where particles are arranged in orderly, repeating patterns and fixed positions
What are Amorphous solids?
Solids where particles lack an ordered internal structure
What are Allotropes?
Different forms of the same element in the same state of matter
What are unit cells?
The smallest portion of a crystal lattice that shows the three-dimensional pattern of the entire lattice
What are the three forms of crystalline carbon?
Diamond, Graphite, and Fullerenes
What are examples of Amorphous solids?
Glass, Plastics, Rubbers, and Asphalt
Phase Diagram Reference

What does a Phase Diagram show?
The circumstances needed for each phase to exist
What is the Triple-Point?
The point where all 3 phases exist in equilibrium
What is the Critical point?
The temperature above which liquid can not exist
What is it called when a solid goes to a liquid?
Melting Point
What is it called when a gas goes to a liquid?
Condensation
What is it called when a liquid goes to a solid?
Freezing Point
What is it called when a solid goes to a gas?
Sublimation
What is it called when a liquid goes to a gas?
Vaporization
What is it called when a gas goes to a solid?
Deposition
What is Dalton’s Law of Partial Pressure?
P(total) = P(A) + P(B) + P(C)…..
What is Boyle’s Law?
Pressure and Volume, inversely proportional

What is Charles Law?
Volume and Temperature, directly proportional

What is Gay-Lussac’s Law?
Pressure and Temperature, directly proportional
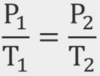
What is the Combined Law?

What Law is shown?

Gay Lussac’s Law
What Law is shown?

Charles Law
What Law is shown?

Boyle’s Law
What is the Ideal Gas Law?

What does all Temperature have to be in and how do you get it?
Kelvin K = °C + 273
What are the over-simplifications of the Ideal Gas Law?
particles take up no space
particles have no attractive forces
When does the Ideal Gas Law not work very well?
High pressure(particles are closer), low Temperature(IMF’s are more noticable, and high Density
What is Effusion?
The escape of gas through small pores
