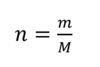Chemical Reactions: Reactants, Products and Energy Change Flashcards
Define chemical reaction.
A process that leads to the transformation of one set of chemical substances to another.
What do chemical reactions involve?
The breaking and formation of chemical bonds.
What can chemical reactions be represented by?
Chemical equations.
Examples of chemical reactions.
Burning Magnesium Iron rusting Acid dissolving Copper Oxide
Define chemical equation.
A symbolic representation of a chemical reaction using formulae.
How are chemical equations set out?
Reactants appear on the left side and products on the right with them separated by an arrow.
Examples of chemical equations.
N₂(g) + 3H₂(g) ➝ NH₃(g)
2Mg(s) + O₂(g) ➝ 2MgO(s)
What do balanced equations indicate?
The relative numbers of particles (atoms, molecules or ions) involved in the reaction.
Define reactants.
The starting materials for a chemical reaction that appear to the left of the arrow in an equation.
Define products.
The substances formed as a result of a chemical reaction that appear to the right of the arrow in the equation.
Define phase change.
A physical change where a substance changes between the solid, liquid, gaseous or aqueous state. Also called a change of state.
Examples of phase change.
Water freezing
Mercury boiling
Salt dissolving
Labelled chemical equation diagram

Define enthalpy (H).
The stored chemical potential energy in a substance (often called the heat content).
What do reactions often involve?
A change in enthalpy
∆H = Hproducts - Hreactants
Example of enthalpy.
Combustion of Ethanol
∆H = -1370kJ
Law of conservation of energy.
The total amount of energy within a system remains constant (is conserved), although the energy within the sysem can be changed from one form to another.
Example of the law of conservation of energy.
Combustion of Ethane.
Energy is released as heat but only because the products have less chemical energy than the reactants.
Law of conservation of mass.
In a closed system mass will remain constant over time.
Mass will only change if matter is added or removed.
Mass (and energy) can neither be created nor destroyed.
Example of the law of conservation of mass.
H2 + O2 → 2H20
4g 32g 36g