What is Huckel’s Rule for aromaticity?
To be aromatic, the molecule must be a planar monocyclic ring with 4n + 2pi electrons.
What makes a stable resonance structure?
Decreased formal charges on atoms and decreased separation of charges.
What is an amine?

What is an amide?

What is a gem-dihalide?
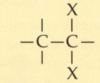
What is a vic-dihalide?

What is an alkoxy?

What is a Ms-mesyl group?
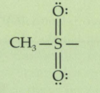
What is a Ts-tosyl group?
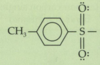
What is a carbonyl?
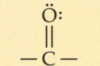
What is a acetyl?

What is an acyl?

What is an anahydride?

What is an aryl group?
Phenyl as a substituent

What is a benzyl?

What is a hydrazine?
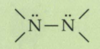
What is a hydrazone?
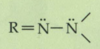
What is a vinyl alkene?

What is a vinyllic alkene?

What is a allyl group?
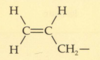
What is a nitrile group?

What is an epoxide?
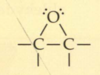
What is a tautomer?

What is an enamine?

What is an imine?

What is an oxime?

What is a nitro group?

What is a nitroso group?

What is an ether?
R—O—R’
What is an ester?

What is ammonium?
protonated amine
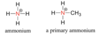
What is amonia?
Simplest amine

What is an anomer?
Stereoisomer that differes at the anomeric carbon of a carbohydrates in CYCLIC FORM (ex: α-D; β-D)
What is an epimer?
A stereoisomer that differs at ONLY ONE stereogenic C (ex: glucose and galactose)
Exception to ionization energy trend with O and N
The ionization energy of N>O because O has electron repulsion from the oxygen’s additional electron in the already half-fills 2p-orbital
Good leaving groups
weak bases in solution
What is a good nucleophile?
strong base
(N is better nucleophile and worse LG than O)
What is an electrophilic addition?
A pi (double/triple) bond is broken and a two sigma (single) bonds are formed.
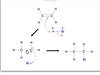
What is a nucleophilic substition?
A nucleophile or an atom with free electrons attacks and electrophilic carbon and relaces the leaving group.

