general functions of the digestive system
- ingest food
- digest food
- absorb nutrients
- defecate waste
how is the rate of delivery of luminal contents coordinated in the GI tract?
- intrinsic nerves: enteric nervous system
- extrinsic nerves: ANS (sympathetic & parasympathetic)
- neurocrine signaling
- paracrine signaling
- endocrine hormones
- solinocrine peptide hormones: secreted into GI lumen before affecting target
functions of digestive secretions
- provide liquid in which solid components are digested
- provide most digestive enzymes
- provides pH balance to intestinal contents
- increases solubility & surface area of lipids to maximize digestibility
- provides lubrication for mass movements in colon
- protection of mucus membranes
what are the components of saliva & their functions?
- serous fluid: moisten food & mucous memebrane
- lysozyme: kill bacteria
- salivary amylase: startch digestion
- mucus: lubricate food; protect GI tract from digesting itself
what is the function of HCl in gastric secretions?
- nonspecific digestion of food
- activate pepsinogen to pepsin
what is the function of pepsin?
digests protein into smaller peptide chains in stomach
secreted by parietal cells as pepsinogen
activated by low pH in stomach lumen
what is the function of bile?
aka bile salts
- emulsify fats, making them available to intestinal lipases
- help make end products soluble & available for absoprtion by the intestinal mucosa
- aid perstalsis
what are the pancreatic digestive secretions?
- trypsin
- chymotrypsin
- carboxypeptidase
- pancreatic amylase
- pancreatic lipase
- ribonuclease
- deoxyribonuclease
- cholesterol esterase
- bicarbonate ions
what are the small intestine digestive secretions?
- mucus
- aminopeptidase
- peptidase
- enterokinase
- amylase
- lipase
what is the function of mucus throughout the GI tract?
- protect mucosa from stomach acid, gastric enzymes, & intestinal enzymes
- provides adhesion for fecal matter
- protect intestinal wall from bacterial action & acid produced in feces
what is the function of trypsin?
digest proteins
breaks polypeptide chains at Arg or Lys residues
what is the function of chymotrypsin?
digests proteins
cleaves carboxyl links of hydrophobic amino acids
what is the function of carboxypeptidase?
digests proteins
removes amino acids from the carboxyl end of peptide chains
what is the function of pancreatic amylase?
digests carbohydrates
hydrolyzes startches & glycogen to form maltose & isomaltose
what is the function of pancreatic lipase?
digests fats
hydrolyzes fats (mostly triacylglycerols) into glycerol & fatty acids
what is the function of ribonuclease?
to digest ribonucleic acid
what is the function of deoxyribonuclease?
to digest deoxyribonucleic acids
hydrolyzes phosphodiester bonds
what is the function of cholesterol esterase?
hydrolyzes cholesterol esters to for cholesterol & free fatty acids
what is the function of bicaronate ions in the GI lumen?
provide appropriate pH for pancreatic enzymes
what is the function of aminopeptidase?
splits polypeptides into amino acids
from amino end of polypeptide chain
what is the function of peptidase?
spolits amino acids from poly peptide chains
what is the function of enterokinase?
activates trypsin from trypsinogen
where is mechanical digestion accomplished?
mouth via mastication
stomach contractions
segmental contraction of intestines
where do the products go following absorption?
water soluble particles travel thru the blood to the liver via portal circulation
fat soluble particles are packaged as micelles & sent in to the lymphatic system via lacteals
where are carbohydrates digested?
- oral cavity via salivary amylase in basic pH into poly- & disaccharides
- duodenum via pancreatic amylase in basic pH in to disaccharides
- lining of small intestine @ brush boarder→ monosaccharides
- lactase
- sucrase
- maltase
- isomaltase
where are proteins digested?
- gastrin lumen via pepsin→ poly- & dipeptides
- duodenum
- trypsin
- chymotrypsin
- carboxypeptidase
- brush border of small intestine
- aminopeptidase
- peptidase
- **last 2→ amino acids, dipeptides, & tripeptides
where are fats digested?
- stomach
- lingual lipase
- gastric lipase
- duodenum w/bile assistance
- lipase
- esterase
- brush border of small intestine
- lipase
**absorbed asa glycerol & fatty acids**
what are 2 substances absorbed in the oral cavity?
nitroglycerin & nictotine
where is skeletal muscle found along the digestive tract?
extreme oral & anal segments
allows for voluntary control at either end
how does the enteric nervous system work?
- stretch is sensed in one section of GI tract
- motor signals behind it & around it stimulate contraction of circular smooth muscle & relaxation of longitiudinal smooth muscle→ contraction of lumen
- motor signal ahead of stretch stimulate relaxation fo circular smooth muscle & contraction of longitudinal smooth muscle→ dilation/expansion of lumen
what is the function of the submucosal plexus?
aka Meissner’s plexus
sensory & motor control of gastric secretions
what is the function of the myenteric plexus?
aka Auerbach’s plexus
sensory & motor control of gastrointestinal motility
how do the myenteric & submucosal pexuses communicate w/each other?
via teriary plexus continuous w/myenteric plexus
deep muscular & mucosal plexuses
coordinate response between 2 major plexuses
what processes are controlled by the enteric nervous system?
- GI tract motility
- constriction of blood vessels
- exocrine & endocrine secretions
describe intrinsic reflex of the circular muscle.
- stretch reception
- ACh & sibtance P from enteric motor neuron→ promote smooth muscle contraction oral to stimulus
- different enteric motor neurons on aboral side release NO & vasoactive intestinal peptide (VIP)→ relax circular muscle
- pressure gradient moves bolus in aboral direction
how does longitudinal muscle respond to stimulus, compared to the circular muscle?
opposite effect, same motor neurotransmitters
- oral: NO & VIP→ relax
- aboral: ACh & Subtance P→ contraction
how does serotonin affect the ENS?
from enteroendocrine cells
released by luminal stimuli
- acts in a paracrine fashion to stimulate ENS interneurons activating the peristalic reflex
ACh effects in digestive system
acetylcholine: parasympathetic & enteric neurotransmitter
- increases motility
- closes sphincters
- increases secretions
substance P effects in digestive system
parasympathetic & enteric neurotransmitter
- increases motility
NO effects in digestive system
aka nitric oxide
parasympethetic & enteric neurotransmitter
- muscle relaxation
NE effects in digestive system
aka norepinephrine
sympathetic neurotransmitter (extrinsic control)
- decreases motility
- decreases blood flow
- decreases secretions
Neuropeptide Y effects on digestive system
sympathetic & enteric neurotransmitter
- decreases motility
- decreases secretion
VIP effects in digestive system
aka vasoactive intestinal polypeptide
enteric neurotransmitter
- induces muscle relaxation
- increases secretions
opoid effect on digestive system
ex. Met & Leu enkephalins
* act indirectly on plexuses to inhibit motility & secretion
GABA effect on digestive system
aka gamma-amino butyric acid
widely expressed in END nerves
- inhibits effects on motility & secretion
epinephrine effect on digestive system
sympathetic neurotransmitter
- inhibits motility indirectly by acting on interneurons of ENS
how does parasympathetic stimulation affect GI function?
- activates motility
- inhibits sphincter tone (relaxes)- allows for more motility
- increases secretions
- no effect on vascular resistance
how does sympathetic stimulation affect GI function?
- decreases motility
- increases sphincter tone→ slows release of food/chyme
- decreases secretions
- except in salivary glands
- increases vascular tone→ vasoconstriction→ decreases blood flow→ less motility
what GI hormones are produced in the stomach?
gastrin & somatostatin
how does cholecystokinin affect GI motility?
increases contractility of gallbladder smooth muscle
how does secretin affect GI motility?
increases movement of gastric juices into the duodenum
what GI hormones are produces in the small intestine?
duodenum or jejunum
secretin, cholecystokinin, motilin, glucose-dependent insulinotropic polypeptide, & somatostatin
what GI hormones are produced by the pancreatic islet cells?
insulin, glucagon, & somatostatin
what GI hormones are made in the colon?
ileum or colon
enteroglucagon & somatostatin
where is ghrelin secreted from?
fundus/upper stomach
where is gastrin secreted from?
antrum of stomach & decreasing incrementally thru to the jejunum
where is cholecystokinin secreted from?
duodenum & jejunum & less so in the ileum
where is secretin secreted from?
duodenum & jejunum & less so in the ileum
where is GIP secreted from?
aka glucose-dependent insulinotropic polypeptide
duodenum & jejunum
where is motilin secreted?
duodenum & jejunum
what signals induce the release of gastrin?
- proteins & amino acids
- stomach distension
- parasympathetic nerve activity
what signal reduce or inhibit gastrin release?
decreased lunimal pH
what signals increase or induce the release of cholecystokinin?
- proteins & amino acids
- fatty acids
- increased parasympathetic nerve activity
what signals increase or induce the release of motilin?
increased parasympathetic nerve activity
what signals decreased or inhibit the release of motilin?
- proteins & amino acids
- carbohydrates
- fatty acids
what signals induce or increase the release of GIP?
aka glucose-dependent insulinotropic polypeptide
- carbohydrates
- fatty acids
what signals induce or increase the release of secretin?
- fatty acids
- decrease in luminal pH
actions of gastrin
act via CCKB receptors
- increases gastric acid secretion
- increases gastric motility
- increases gastric mucosal growth
- decreases gastric emptying
- maximizes stomch churning
actions of secretin
- increases pancreatic & biliary bicarbonate secretion
- decreases gastric acid secretion
actions of cholecystokinin
- increases pancreatic enzyme secretions via CCKA
- increases gallbladder contraction via CCKA
- decreases sphincter of Oddi tone indirectly
- decreases gatric emptying via CCKB
actions of GIP
aka glucose-dependent insulinotropic polypeptide
- increases pancreatic insulin secretion
- decreases gastric acid secretion
- decreases gastric emptying
actions of Motilin
increases gastric & intestinal motility
actions of Ghrelin
- increases growth hormone secretion
- increases feeeding
- increases weight gain
define ingestion
voluntary intake of food into the oral cavity
what important function is performed by the lips & taste buds?
sense food’s temperature & composition
why is absorption limited in the oral cavity?
- materials don’t stay in there very long
- very small surface area
- substrates must be absorbable, but most need to undergo digestion into monomers first
what is the composition of saliva?
- watery hypotonic fluid
- ions: Na+, HCO3- (pH balance), Cl-, K+
- mucus
- digestive enzymes: salivary alpha-amylase & ligual lipase
- lysozyme
- peroxidases
- antibodies
what are the functions of saliva?
- maintenance of oral hygiene
- lubrication for speaking
- swallowing
- digstive function: amylase & lipase
- salivation to taste food & to moisten dry foods
what are the functions of mucus?
- traps bacteria
- creates “unstirred layer” to protect epithelial cell from acid and/or digestive enzymes in lumen
- lubricant
what component of mucus give its gel-like characteristic?
gycoproteins
how does saliva differ at its origin in the secretory endpieces?
isotonic serious fluid in endpieces
NaCl is removed & K+ and HCO3- are added along ducts
produces hypotonic saliva
how can salivary blood flow be increased?
- kallikrein: paracrine enzyme from acinar cells
- parasympathetic ACh & VIP vasodilators
- local vasodilators
why do methanphetamine addicts experience severe tooth decay?
dry mouth
no protective properties of saliva
what are the major effects of adrenergic stimulation on salivary secretion & the receptor types involved?
- norepinephrine released from sympathetic nerve terminals
- alpha-adrenergic:
- 2nd messenger: inctracellular calcium
- greater fluid over protein secretion
- beta-adrenergic:
- 2nd messenger: cAMP
- greater protein over fluid secretion
what effects does the parasympathetic nervous system have on salivary secretion?
- ACh
- receptor: M3
- 2nd messenger: calcium
- response: greater fluid over protein secretion
- substance P
- receptor: tachykinin NK-1
- 2nd messenger: calcium
- response: greater fluid over protein production
why is it okay that reabsorption efficiency is lost through the salivary ducts during faster salivary flow?
becuase when flow is fast, swallowing is likely
thus NaCl will be reabsorbed elsewhere instead of lost
what hormones stimulate sodium reabsorption?
aldosterone & anti-diuretic hormone (ADH)
how does flow rate of saliva affect is composition?
- at high flow, Na+ & Cl- reabsorption is decreased
- at high flow, HCO3- & K+ secretion is increased
- HCO3- greatly
- K+ just keeps up for normal concentration
low flow: [Na+ & Cl-] < [HCO3- & K+]
high flow: increased [Na+ & Cl- & HCO3-]
which gland secretes salivary alpha-amylase?
acinar cells of parotid & submandibular glands
which gland secretes lingual lipase?
acinar cells of sublingual gland
which gland secretes kallikrein?
acinar (and maybe ductal) cells of parotid, sublingual, & submandibular glands
where is lingual lipase active?
secreted in the mouth
but optimal pH= 4.5-5.4, similar to a food-filled stomach
what is kallikrein?
serine protease
secreted by acinar cells in all three major salivary glands
paracrine function to increase bradykinin production at salivary arterioles→ increases blood flow to glands→ increased salivary flow rate
what is xerostomia & its cause?
dry mouth
common w/increasing age
can be caused by pharmaceutical agents (tricyclics & sympathomimetics)
can cause difficulty swallowing & speaking
What is Sjogren syndrome?
autoimmune disorder
attaches salivary & lacrimal glands
leads to obstructed ducts
symptoms: dry mouth, superficial oral ulcerations, poor dentition, & dry eyes
what is sialolithiasis?
formation of Ca2+ stones in salivary glands
most frequently found in submandibular gland
frequently associated w/chronic infection of the glands, dehydration, Sjogren’s syndrome, &/or increased local levels of Ca2+
what is the function of the pharynx?
transfer of food to esophagus
what is the function of the upper esophageal sphincter?
allow entry of food into esophagus
protects airway from swallowed material
protects airway from gastric reflux
what is the function of the esophagus?
transport bolus form pharynx to stomach
clear material refluxed from stomach
what is the function of the lower esophageal sphincter?
alloes entry of food into stomach
protects esophagus from gastric reflux
what controls the swallowing process beyond the oral stage?
swallowing center in medulla & lower pons
stimulated by touch receptors in oropharynx
what are the stages of swallowing?
- oral phase: tongue moves bolus into place
- pharyngeal: soft palate covers nasopharynx & tongue forces food up & back then down
- UES relaxes: epiglottis desciends to cover larynx & trachea
- peristalsis wave intiated
why is the pressure in the UES higher than atmospheric?
rests at 40mmHg
b/c it is usually closed as breathing is repressed when it is open
skeletal muscle subjected to enough motor tone to keep it closed
relaxes to just below atmospheric during late pharyngeal stage to allow bolus in
after bolus passes, pressure rises above resting to propel food down esophagus
how does the LES compare to the UES?
LES is smooth muscle instead of skeletal
tonic pressure of 20mmHg
pressure drops to 0mmHg as food approaches from oral direction
stays at 0mmHg for several seconds
what controls the opening of the LES?
vagal inhibitory fibers via vasoactive intestinal peptide (VIP) & NO neurotransmitters
how does the LES remain closed?
constant ACh & substance P release from vagal excitatory fibers (VEF)
how does rabies affect the digestive system?
- extreme excitability of swallowing center in CNS→ pharyngeal contracture at thought of swallowing→ hydrophobia
- hypersalivation
what causes heartburn?
transient relaxations of the LES
allowing stomach acid to enter the esophagus
some chemical agents can reduce esophageal barrier function and/or prolong duration of relaxation
this manometer trace is…

achalasia
this manometer trace is…
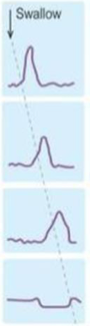
normal
signs & symptoms of achalasia
regurgitating undigested food at night
difficulty swallowing solids & liquids
“fullness”in chest
often confused w/angina
may lose weight
cause of achalasia
idiopathic destruction of nitrergic neurons of myenteric plexus→ functional narrowing of lower esophagus & peristaltis failure
dilated esophagus proximal to narrowed gastroesophageal junction
failure of LES to relax or impaired peristalsis in distal esophagus
what are the types of dysphagia & their causes?
- oropharyngeal: abnormalities of muscles, nerves, or structure of oral cavity, pharynx, or UES
- esophageal: mechanical or motility problem of body of esophagus, LES, or cardia of stomach
what controls gastic motility in general?
autonomic & hormonal control
how is chyme moved into the duodenum?
pressure gradient
what allows for stomach expansion?
rugae on the inner surface of the stomach
where is the orad?
first 2/3 of stomach body
where is the caudad?
last 1/3 of body + antrum of stomach
where is the pacemaker zone of the stomach?
on greater curvature near border of fundus & orad
3-4 slow waves/min
where is the greatest capacity for expansion in the stomach?
the fundus
what controls pyloric openign & closing?
stomach factors open it & duodenal factors close it
doesn’t open more than 1-2mm during gastric emptying
what is receptive relaxation?
the amount of material the stomach can accomodate w/o significant pressure changes
typically 1.5L
what are the methods for bariatric sx?
elastic band at cardiac region
vertical sleeve
gastrectomy
gastric bypass
what are the physiological effects of bariatric sx?
reduced capacity for stomach acid secretion
reduced gastric hormone secretion
how does gastric peristaltic waves change w/& w/o food?
fed: every 15-20 econds or 3-4 waves/minute
fasting: inactive 75-90 minutes, then 5-10 minutes of intense motility
empty 12-24 hours: hunger contractions begin & peak in intensity at 3-4 days then weaken
in what order do food types enter the duodenum from the stomach?
liquids
carbohydrates
proteins
lipids (b/c they float to the top in the stomach)
what is antral systole?
rhythmic contractions of antrum (distal stomach)
what is retropulsion?
when food particle are forced against the resistant pyloric sphincter & reflected back into the stomach
mechanically breaks down food
chyme
suspension of partially dissolved particles
enter duodenum via pyloric sphincter 2-4mL at a time
what are the intrinsic regulatory mechanisms of gastric emptying?
- gastric stretching→ relaxation of pyloric sphincter
- duodenal stretching→ constrict the pyloric sphincter
what are the extrinsic regulatory mechanisms of gastric emptying?
- Parasympathetic inceases gastric empyting
- ACh increases gastric motility & thus pressure
- VIP relaxes pylorus
- gastrin-releasing peptide causes endocrine secretion of gastrin
- sympathetic decreases gastric emptying
- via stimulating pyloric contraction
what is the ileal brake?
nutrients in terminal ileum & cecum
lead to peptide endocrine hormones that slow gastric emptying
peptide YY & glucagon-like peptides are thought to be involved
what duodenal factors can decrease gastric emptying?
- duodenal distension
- presence of nutrients (especially fats)
- need time to be enzymatically digested
- acidity/increased [H+] in duodenum
- releases enterogastrones that inhibit gastric emptying & secretion of gastric acid
define enterogastrone.
any substance released from duodenum that inhibits movement of chyme towards anus
ex. secretin (pH response) & CCK (nutrient response)
what is the primary mechanism to optimize nutrient digestion & absorption?
ileal brake
fucntions of gastric secretions
- digestion of peptides & lipids
- protection of stomach epithelium
- absorption of vitamin B12
- destroy bacteria & other microorganisms
what is normal stomach pH
1.0-3.5
what protects gastric epithelium?
mucus & bicarbonate
- secretion of alkaline fluid (bicarbonate) by surface epithelia
- secretion of mucus
- prostaglandins: decrease acid secretion, increase mucosal flow, & increase HCO3- & mucus secretion
- adequate blood flow
- growth factors ensure replacement of damaged cells
what pharmalogical agents inhibit mucosal secretions?
NSAIDS & alcohol
how does gastric juice composition vary with flow rate?
[H+] starts low & increases exponentially
[Cl-] starts high & increases gradually
[Na+] starts high & decreases rapidly
[K+] starts very low & increases slightly
how do parietal cells secrete HCl?
via the tubulovesicular system
- proton pumps & Cl- channels are internalized in cytosolic tubulovesicles
- increases in cAMP & Ca2+ promote trafficking of Cl- & tubulovesicle fusion w/apical cell surface
- creates pattern of secretory tubules extending w/in cell
why is it important that chief cell are located deeper in gastric pits than parietal cells?
they usually secrete at the same time
non-acidic cheif cell secretions provide pressure gradient to drive acid from parietal cells into main gastric lumen
what signals stimulate parietal cell acid secretion?
ACh via M3 receptor
Gastrin via CCKB receptor
Histamine via H2 receptor
*all act directly thru calcium & cAMP-dependent signaling pathways*
also: protein, smoking, caffeine, stress, alcohol, tobacco
what signals inhibit acid secretion by parietal cells?
somatostatin via SSRT1 receptor
prostaglandins (mostly PGE2 & i2) via EP3 receptors
also: stomach acid (negative feedback) & sleep
what are the phases of acid secretion & their driving factors?
- cephalic: before food enters stomach (30%)
- sight, smell, thought or taste of food
- greater appetite, greater stimulation
- gastric: triggered by stomach distension (60%)
- food entry decrease luminal [H+]
- distension stimulate gastrin which stimulates acid secretion
- intestinal: duodenal G cell release of gastrin (10%)
- due to duodenal distention
actions of Guanylin & where it is secreted
from colon
released upon eating a salt load
promotes sodium loss from kidneys
actions of uroguanylin & where it is secreted
from duodenum & proximal jejunum
released upon eating a salt load
promotes sodium loss from kidneys
actions of pancreatic polypeptide & where it is secreted
released from F cells in pancreas upon ingestion of a meal
inhibits pancreatic secretion of enzymes & bicarbonate
**role undefined**
actions of peptide YY & where it is secreted
released from distal ileum in response to a meal
decreases GI motility
decreases gastric & intestinal secretions
actions of GLP & where it is secreted
aka glucagon-like peptide or enteroglucagon
released from mucosal L cells in terminal ileum & colon
in response to luminal sugars
enhances GIP release & inhibits glucagon release
what factors influence gastric lipase secretion?
stimulates: gastrin
inhibits: glucagon-like peptide 1 & cholecystokinin
when are preduodenal lipases important?
- as neonates b/c pancreatic lipases don’t work well on milk fats
- pts w/pancreatic insufficiency
what substaces are absorbed in the stomach?
ethanol
NSAIDs
short to medium chain fatty acids
what are the causes of vomiting?
gastric irritation
vestibular function/stimuli (motion sickness)
gastric outlet constriction
function of vomiting
means by which the upper GI tract rids itself of its contents when any part becomes excessively irritated, over distended, or over excitable
especially from the duodenum
vomiting reflex
controlled by vomiting center in medulla
coordinated w/medullary respiratory centers to prevent choking on vomit
- pyloric sphincter & stomach relax to receive intestinal contents
- sudden increase in intraabdominal pressure drives gastric contents into esophagus
- in retching, UES doesn’t open
- if UES opens, then vomitus is projected into oral cavity
- epiglottis is tightly closed
main functions of the large intestine
fluid absorption & storage and elimination of fecal waste
most water absorbed in cecum, ascending colon, & first portion of transverse colon
what occurs to dietary fiber in the large intestine?
some but not all undergoes fermentation by gut flora (bacteria)
products are short chain fatty acids (acetate, propionate and butyrate) & get absorbed
fatty acids create more acidic colonic fluids that prevent bacterial overgrowth
what are the main components in feces?
non-startch polysaccharides like cellulose (dietary fiber)
bacteria
inorganic material
in a small volume of K+-rich fluid
what are the motility patterns in the gastrointestinal tract?
- fasting: migrating motor complex
- digestive: stationary mixing & perstaltic
- mucosal folding: alters surface of mucosal layer
what is the purpose of mucosal folding suring digestion?
mucosal folding is altered by the muscularis mucosae smooth muscle layer of the mucosa
alters the surface layer of mucosa for luminal exposure
movements also pump lacteals empty
describe the migrating motor complex
electrical control driving strong rhythmic contractions of stomach & small intestine during fasting state
very strong form of perstalsis
typically occurs at intervals of 90-120 minutes
- prolonged quiescent period
- increasing action potential frequency & contractility
- peak electrical & mechanical activity
clears small intestine of residual content
what is the purpose of the migrating motor complex?
clear small intestine of residual contents, including: undigested food, bacteria (or it would overgrow), desquamated cells, intestinal & pancreatic biliary secretions
what is the underlying electrical mechanism behind the migrating motor complex?
slow waves of electrical impulse from the pacemaker region of the stomach travels through the small intestine
only slow waves that produce a spike produce muscle tension
typically 12 slow waves/min in duodenum & 8-9 slow waves/min in the ileum
what are ICCs?
interstitial cells of Cajal
specialized interstitial multipolar cells associated w/all regions of the gut from which slow waves are recorded
branched processes connect ICCs to each other & form a network aroudn the myenteric plexus
also communiate w/smooth muscle cells via gap junctions
direct interaction w/smooth muscle cells
what is the purpose of segmentation?
- acid & bicarbonate can interact w/carbonic anhydrase to create proper pH for enzymatic activity
- enzymes & substrates can interact
- residual multimolecule particles are torn apart
- other materials (bile salts & colipases) can interact w/enzymes & substrates
- materials in luminal center can become lodged in unstirred layer near intestinal wall for final digestion & absorption
what is the rate of peristalsis in the fed state?
1cm/min in the small intestine
what is the gastro-enteric reflex?
filling of the stomach induces increased motility of teh intestines downstream
requires ANS in distal locations like the ileum
what is the gastro-ileal reflex?
specific effect on the ileum
promotes emptying of ileum into cecum via ileocecal sphincter
requires ANS stimulation
what is the gastro-colic reflex?
promotes mass movements or defecation after a meal
requires ANS input
what is the duodeno-gastric reflex?
decreases motility proximally in stomach
what is the ileogastric reflex?
decreases motility proximally in stomach
requires ANS signalling
what is the colonogastric reflex?
extreme distension in one part of the small intestine promotes relaxation of the rest of the intestine & movement
overall motility is increased
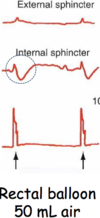
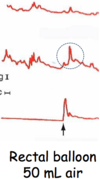
what is the recto-sphincteric reflex?
relaxes internal anal sphincter
required for defecation
what hormones increase small intestinal motility?
gastrin, cholecystokinin, & serotonin
what hormones decrease small intestinal motility?
secretin & glucagon
what are the roles of the ileocecal valve?
regulates chyme flow into the colon from the ileum
protection against movement of bacteria from colon into the small intestine
how is chyme movement into the cecum regulated?
via the ileocecal valve
one-way flutter valve controlled by smooth muscle ring
cecal contraction colonic distension increases its tone (closes)
increased ileal contraction & distension causes sphincter relaxation
mediated by ENS
what is the functional purpose to haustrations?
to facilitate concentration of a liquid chyme into semi-solid mass
how do haustrations in the colon differ from segmentations in the small intestine?
longitudinal muscle organization
both controlled by slow waves
haustrations induced by longer more intense but less frequent slow waves (3-7/min)
haustrations also promoted by ACh from PNS (vagal & sacral)
how does the sympathetic nervous system affect colonic motility?
inhibits motility via mesenteric & hypogastric plexuses
what is the speed of peristalsis in the the colon?
5-10cm/hour
describe the mechanism behind mass movements
mass movements occur in sigmoid thru rectum
1-3 times/day
- constrictive ring appears in response to distended or irritated section of colon
- area 20cm distal loose haustrations
- longitudinal muscle constricts
- forces mass of material in distal direction
- if enter rectum, feel desire to defecate
what controls defecation?
relaxation of internal anal sphincter, secretion of lubricating mucus into rectum & anal canal, and mass movement are all controlled parasympathetically
BUT relaxation of the external anal spincter & increasing abdominopelvic pressure are voluntary muscle controls
Hirschsprung’s Dz
ENS excititory & inihibitory neurons from myenteric & submucosal plexuses are absent from a egment of distal colon
segment is narrowed due to tonic contraction
feces accumulate proximally & megacolon is formed from distension
measurements of …
normal colon
measurements of…
Hirschsprung’s dz colon
how much intestinal secretion are there per day?
1.5L
what are the types of intestinal secretions?
- NaHCO3
- duodenum, mostly
- neutralizes stomach acid
- secreted from Brunner’s glands & enterocytes
- NaCl
- via CFTR channel protein in enterocytes
- trhoughout small intestine
- osmotic equilibration of gut contents
- flush pathogens from gut
net fluid movement across the gut & its driving forces
Jnet = Jabs - Jsec
movement can occur via trans- or paracellular routes
secretion is driven by secretion of Cl- & HCO3-
absorption is driven by Na+-dependent nutrient absorption
what factors increase intestinal secretions?
- submucosal release of ACh & VIP
- local reflexes (distension)
- parasympathetic input thru the ENS promotes secretion of electrolytes & water
what factors inhibit intestinal secretion?
submucosal release of neuropeptide Y
sympathetic input thru the ENS
how does controling the CFTR receptor control all secretions?
CFTR controls rate of Cl- secretion
Na+ secretion is driven by Cl-
H2O secretion is driven by Na+
the aqueous component of colonic secretions is rich in…
K+ & HCO3-
why is bicarbonate needed to protect colonic mucosa?
like elsewhere to protect from low pH
low pH in colon is caused by bacterial metabolism
why is cholera life threatening?
secretes 2 toxins
one increases cAMP→ activates CFTR→ increased Cl- secretion→ increases Na+ secretion→ increases H2O secretion
another makes tight junctions leakier→ increases Na+ & H2O secretion
diarrhea can reach 1L/hr→ hypovolemic shock, hypokalemia, loss of bicarbonate (causes non-respiratory acidosis)
what is the function of pancreatic ducts?
transport of pancreatic enzymes
secretion of HCO3- & water
how do the concentrations of ions in pancreatic secretions vary with flow rate?
Na+ is stable at a high concentration & K+ is stable at a low concentration
Cl- concentration decreases with increasing flow
HCO3- concentrations increase w/increasing flow
mechanism of pancreatic secretion
- acini produce isotonic fluid w/enzymes
- ductal system secretions HCO3- & absorb Cl-
what are the mechanisms of secretory control in the pancreatic acinar cells?
- zymogen are stored in granules at apical pole
- exocytosed upon activation of cAMP or Ca2+-dependent pathways triggered by hormones & neurocrine agents
- fluid secretion stilumated by ACh, secretin, VIP
- protein secretion by CCK & ACh
what receptors activate the Ca2+-dependent pathway?
muscarinic M3 & CCKA
what receptors activate the cAMP-dependent pathway?
SCTR (secretin) & VPAC2 receptor (VIP)
secretin acts mostly on the acini or the ducts?
ducts
what hormone preferentially stimulates pancreatic ducts?
secretin
how is exocrine pancreatic secretions controlled during different phases of digestion?
- cephalic: sight, smell, taste of food→ vagal & eNS stimulation of acinar & ductal cells
- gastric: distentsion of stomach→ vagovagal & gastropancreatic reflexes stimulate acinar & duct cells
- intestinal:
- acid in duodenum→ secretin stimulates ductal cells (HCO3-)
- amino acids, fatty acids, & Ca2+→ CCK stimulates afferent arm of vagovagal reflexes to acinar & ductal cells
- distension of duodenum or hypertonicity in duodenum→ enterohepatic reflex stimulate acinar & ductal cells
- mediated by ACh, CCK, & VIP
when is CCK secreted?
when free fatty acids and certain amino acids are present in the lumen
secreted by I cells
how do CCK-RP & MP differ?
CCK-RP= cholecystokinin-releasing peptide
MP= monitor peptide
CCK-RP requires nutrients to stimulate its release, but MP does not
where is monitor peptide secreted from?
solinocrine secretion from the pancreatic acinar cells into pancreatic juice
where is CCK-RP secreted from?
CCK-RP= cholecytokinin-releasing peptide
solinocrine factor released from enterocytes in presence of free fatty acids
where is pancreatic alpha-amylase active?
lumen of small intestine
what are the carbohydrate digestive enzymes found in the brush border?
hydrolases: sucrase-isomaltase complex, glucoamylase (maltase), lactase
what limits monosaccharide uptake?
availability of specific membrane transporters
how are the monosaccharides (glucose, galactose, & fructose) absorbed?
Fuctose→ GLUT-5 (facilitated diffusion)
glucose & galactose→ Na+-linked symporter SGLT (all 3 together)- secondary active transporter
how do the monosaccharides enter the blood?
transport via GLUT2 in basolateral enterocyte membrane
how are the majority of amino acids absorbed?
via Na+-dependent cotransport of single amino acids OR SLC solute carriers that transport a subset of amino acids
how are di- & tripeptides absorbed?
PepT1
peptide transporter 1
symporter driven by H+gradient
most important for Gly & Pro b/c proteins rich in these amino acids are slow to digest
**digested by intracellular peptidases**
what is the “kinetic advantage” of amino acids?
PepT1 allows for a faster rate of amino acid entry into the blood since they are being absorbed faster than one at a time
Hartnup’s dz
autosomal recessive dz
small intestine & renal tubule abnormalities in absorption of neutral amino acids
PepT1 compensates for amino acid absorption→ amino acid absorption is normal
Cystinuria
autosomal recessive dz
small intestine & renal tubule abnormalities in absorption of basic amino acids
PepT1 compensates for amino acid absorption→ amino acid absorption is normal
lysinuric protein intolerance
autosomal recessive disorder
impaired cationic amino acids transport across basolateral membrane
Arg, Lys, ornithine
SLC7A7 gene mutation
how can colonic water absorption be increased?
aldosterone
increases absorption of Na+ too
accomplished via increasing ENaC expression in the colon
why is the apical ENaC & basolateral Na+/K+ ATPase pump system important in the colon?
ENaC= eletrogenic sodium channel
creates electrical gradient that drives paracellular absorption of Cl-
this is what drives water absorption
what does the proximal colon use for energy?
short-chain fatty acids (acetate, propionate, & butyrate)
from bacterial metabolism
uptake by Na+-dependent symport: driven by low intracellular Na+
what is K+ status in the colon?
contrasting the passively absorption of the rest of the gut
K+ is secreted via K+-selective channels
how does vitamin D affect divalent ion absorption?
hormone cholecalciferol, which is made from vitamin D, increases production & translocation of calcium ion channels to the apical membrane
Mg2+ uses the same ion channel
of channels is rate-limiting for their absorption
what is the mechanism of action for milk of magnesia?
excessive magnesium has limited absorption
increased chemical gradient to luminal side
water retained in feces
thus it acts as a stool softener
what happens to Fe3+ stored in the enterocytes?
if unused, aka transported into the blood, then it will go back into the lumen when the enterocyst sloughs off in a number of days
where is ascorbic acid absorbed?
ileum
where is biotin absorbed?
duodenum & jejunum
where is choline absorbed?
small intestine
where is folate absorbed?
jejunum
whre is inositol absorbed?
small intestine
where is nicotinic acid absorbed?
jejunum
where is pantothenic acid absorbed?
small intestine
where are the B vitamins absorbed?
- B1 (thiamine): jejunum
- B2 (riboflavin): duodenum & jejunum
- B6 (pyridoxine): duodenum & jejunum
- B12 (cobalamin): distal ileum
what is meconium ileus?
obstruction of small intestine in newborn caused by impaction of thick, dry meconium
insufficient fluid secretion & lack of functional trypsin
cardinal sign of cystic fibrosis
90% of cases are due to cystic fibrosis
abd distension, vomiting, failure to pass meconium w/in 24-48hrs after birth, & rapid dehydration w/assoc. electrolyte imbalance
what materials are stored in the liver?
- protein
- glycogen
- vitamin A
- vitamin D
- vitamin B12
- folic acid
- iron
what is produced in the liver?
- plasma proteins
- glycogen
- phospholipids
- heparin
- bile acids
- bile pigments
what metabolic functions are performed by the liver?
- carbohydrates
- glyconeogenesis, glycogenesis, & glycogenolysis
- proteins
- synthesis
- deamination & transaminations
- urea formation from nitrogen
- fats
- synthesis of: triglycerides, phospholipids, & ketone bodies
what detoxification is performed by the liver?
- excretion of heavy metals
- excretion of cholesterol
- excretion of bile pigments
- excretion of drugs
- excretion of hormones
- detoxification of toxic substances & conjugation
function of bile
make lipid digestion more efficient
emulsify lipids
allows for excretion of heavy metals, fat-soluble drugs, cholesterol, etc.
why are there two types of bile? compare & contrast them.
- yellow bile comes directly form the liver
- serous→ greater volume
- green bile comes from the gallbladder
- osmotically equivalent
- fewer Na+ & Cl-
- more bile acids
- both work equally well
- water concentration is equal, but volume is greatly reduced from gallbladder
composition of bile
- bile salts
- bile pigments (ex. bilirubin)
- cholesterol
- lecithin (phosphatidylcholine)
- HCO3-
- Ca2+
- Na+
- K+
- Cl-
- water
pH=8-9
how much bile is excreted daily?
typically 500mL, but up to 1L
what are the types of bile acids?
- all derived from cholesterol
- each modification makes them more water-soluble
- primary: made in liver
- secondary: modified by intestinal & colonic bacteria
- tertiary: modified by hepatocytes after recycling via enterohepatic circulation
describe the cellular mechanism of bile-dependent formation.
- Na+/K+-ATPase in basolateral membrane maintains gradient for Na+-dependent uptake of bile salts across sinusoidal membrane
- bile salts conjugated w/glycine or taurine in sER
- secreted into biliary canaliculus
- mostly by ATP-dependent transporters
- Na+ passes paracellularly thru tight junction from basolateral side into canaliculus
- water follows Na+
describe the cellular mechanism of bile-independent formation.
water flows into canaliculus due to accumulation of other osmotically active solutes (HCO3-) which exits hepatocyte via transporter in canalicular membrane
what dilutes canalicular bile & why?
water & bicarbonate
bicarbonate makes bile more alkaline
thus reducing risk of Ca2+ precipitation
what hormone affect the cholangiocyte secretions?
cholangiocyte= bile ductular cells
secretin & VIP increase secretions
somatostatin decreases secretions
what controls bile release into the duodenum?
- ACh from ENS during cephalin phase→ mild rhythmic contractions of gallbladder
- entry of chyme into duodenum→ CCK release from I cells→ powerful contraction of gallblader smooth muscle & indirectly relaxes sphincter of Oddi
- intestinal phase, secretin release→ stimulated pancreas & cholangiocyte secretion of HCO3-
- sphincter of Oddi relaxed via NO & VIP released from ENS after CCK stimulation
how much bile salts are recycled?
95% of secreted bile is returned to the liver per pass
20% of bile acid pool is replaced thru new synthesis daily
how are bile salts/acids recovered?
reabsorbed in terminal ileum
- carrier-mediated absorption in terminal ileum
- deconjugation to primary bile acid before being absorbed passively or actively
- conversion of primary bile acid to secondary bile acid w/subsequent absorption of deoxycholic acid
- passive diffusion along small intestine
why are tertiary bile acids not reabsorbed?
because they are more water soluble than fat-soluble
thus they are excreted
they are also potentially cytotoxic
what are chylomicrons?
triglycerides, cholesterol, & phospholipids aggregated together in enterocytes
transported to lacteal for lymph transport
steatorrhea
pale, malodorous stool
caused by high concentration of fats in feces
early warning sign of cystic fibrosis
where does renal filtration occur?
glomerulus
what are the main functions of the kidney?
- clearance of blood waste products
- urea, uric acid, creatin, drugs
- regulation of H2O & eletrolytes
- hormone secretion
- erythropoietin & renin
- activation of vitamin D
inlets & outlets of kidney
inlet: renal artery
outlets: renal vein & renal pelvis/ureter
what is the functional unit in the kidney?
nephron
parts of nephron from from afferent arteriole
- Bowman’s capsule
- glomerular capillary
- Bowman’s space
- proximal convoluted tubule
- proximal straight tubule
- thin descending limb of the loop of Henle
- thin ascending limb of the loop of Henle
- thick ascending limb of the loop of Henle
- macula densa
- distal convoluted tubule
- connecting tubule
- late distal tubule
- late distal tubule
- initial collecting tubule
- cortical collecting tubule
- medullary collecting tubule
- outer & inner
why does the nephron have two capillary beds?
- glomerular capillary
- arterial
- filtration only
- afferent arteriole to efferent arteriole
- peritubular capillary bed
- absorption, secretion, & reabsorption
- efferent arteriole to renal vein
- more thorough cleaning system
- throw it all away and recollect whats needed
how much blood is sent to the kidneys?
20% of CO
normally 1L/min
where is water absorbed in the nephron?
2/3 in proximal tubule & 1/3 in distal tubule
in normal healthy circumstances, how much blood is filtered by the kidneys/min? /day?
120mL/min & 180L/day
- 20% of CO= 1L/min
- only plasma is filtered
- blood is 55% plasma=600mL
- only 20% of plasma that travels thru glomerulus is filtered= 120mL/min
- 120mL/min * 60min/hr *24hr/day * 1L/1000mL= 180L/day
where does fine regulation of water & electrolytes take place in the nephron?
late distal tubule & collecting tubule
where do hormone exert most of their effects on the nephron?
late distal tubule & collecting duct
where is the nephron impermeable to water?
ascending limb of loop of Henle & early distal tubule
what does facultively permeable mean?
permeable when facilitated
discretionarily permeable (ie hormonal or neuronal actions)
what controls urine volume?
not GFR
reabsorption process in distal tubule
RPF
renal plasma flow
RPFa= renal plasma flow entering kidney
RPFv= renal plasma flow leaving kidney
RPFa=RPFv in healthy kidneys b/c 99% is reabsorbed
how is the amount of substance in the unrine calculated?
urine flow (Vdot) /min * concentration of substance in urine (U)
also known as the excreted amount
how is the cleared amount calculated?
cleared is plasma cleared of substance
RPF (Pa - Pv) = Vdot * U
where Pa= arterial concentration of substance & Pv= venous concentration of substance
define clearance
volume of plasma that is cleared of a substance in a unit of time
how is clearance calculated?
C = (Vdot * U) / Pa
units are mL/min
how can clearance be measured?
measure urine excretion in a certain time frame, concentration of substance in urine & plasma
but this is dependent on plasma concentration being constant during the entire collection time period, ex. equilibrium or infusion of substance over a long period
GFR
glomerular flow rate
flow rate of fluid thru the kidney
what is the filtered load?
GFR * Pa
substance must be freely filtered
Msdot
amount of substance secreted from perilobular capillary into kidney tubule
per time unit
Mrdot
amount of substance reaborbed from kidney tubule to perilobular capillary
per time unit
how is the excreted amount of a substance calculated?
Vdot * U = GFR * Pa + (Msdot -Mrdot)
but when a substance is neither secreted or reabsorbed GFR=C= (Vdot * U)/Pa
what is the clearance of inulin?
C= 120mL/min= GFR
b/c no excretion or absorption b/b it doesn’t penetrate cell membranes
for what substances does GFR= C?
inulin and approximately creatinine
how can creatinine clearance be estimated?
from plasma concentration b/c very little is secreted or absorbed
when does C=RPF?
RPF= renal plasma flow
when substance is totally secreted
C= 600mL/min (all plasma to kidney/min)
ex. para-amino hippuric acid
excreted amount is a sum of?
filtrated substance + secreted substance
why does the secretion curve plateau?
b/c substance transport mechanism has been maximized
aka transport proteins have been saturated
why does the secretion curve curve instead of make a sharp angle at the point of plateau?
because it is graphed for the kidneys, summation of all 2 million nephrons instead of a single nephron
why is C=0 for glucose in healthy individuals?
because it is all reabsorbed
when is C >0 for glucose?
when reabsorption is maximized at 375mg/mL
what does fractional excretion tell us?
whether a substance is reabsorbed or secreted independent of the underlying mechanism
<1→ net reabsorption
>1→ net secretion
how is fractional excretion calculated?
Cx/Cinulin ≈ Cx/GFR
how is filtration fraction calculated?
FF= GFR/RPF
healthy kidneys FF= 120mL/min / 600mL/min = 20%
what is the kidney capsule made of?
outer layer is fibroblasts & collagen
inner layer is myofibroblasts

what forms the renal medulla?
8-12 conical renal pyramids
what separates the renal pyramids from each other?
renal columns
extensions from renal cortex
what is a renal lobe?
medullar renal pyramid w/it’s surrounding cortical tissue at base & sides
can be seen w/o a microscope
what is a kidney lobule?
group of nephrons around a single medullary ray & all drain into a single collecting duct
not visible w/o a microscope
renal papilla
tip of each renal pyramid that projects into a minor calyx
renal pelvis
considered expanded part of ureter
branches to form major & minor calyces
what is the funtional unit of the kidney?
uriniferous tubule = nephron + collecting duct
what separates uriniferous tubules?
loose connective tissue of the basal lamina
what are the different types of nephrons and how can they be differentiated?
majority are cortical nephrons, mostly located in the cortex (tip of loop of Henle & collecting duct enter medulla)
1:7 are juxtamedullary nephrons: long loops of Henle that extend into medulla→ crucial for production of concentrated urine
where are vasa recta found in the kidneys?
only around the juxtamedullary nephrons
function of vasa recta
participate in countercurrent exchange
production of concentrated urine
what is the blood flow pathway through the kidneys?
- renal artery
- segmental artery
- interlobar artery
- arcuate artery
- interlobular artery
- afferet arteriole
- glomerulus
- efferent arteriole
- peritubular capillaries/vasa recta
- interlobular vein
- arcuate vein
- interlobar vein
- renal vein
what forms the renal corpuscle?
glomerulus: 10-20 capillary loops
surrounded by Bowman’s capsule: double-layered epithelial cup
what cell type forms the visceral layer of Bowman’s capsule?
podocytes
they wrap around glomerular capillaries
what is a pedicel?
foot-like process of a podocyte that wraps around glomerular capillaries
function of nephrin
protein between pedicels of podocytes
forms slit diaphragm that allows filtration of water & sugar, but not proteins
where are mesangial cells found?
between capillaries in glomerulus
function of mesangial cells
- physical support: cover capillary surfaces not covered by podocytes
- contraction: respond to vasoactive substances to help maintain hydrostatic pressure for optimal flow rate
- secretion: synthesize & secrete cytokines, prostaglandins, etc. for immune defense
- phagocytosis: of protein aggregates that adhere to glomerular filter
what are lacis cells?
extraglomerular mesangial cells
function of the juxtaglomerular apparatus
feedback mechanism for autoregulation of renal blood flow to keep GFR relatively constant
components of juxtaglomerular apparatus
macula densa
juxtaglomerular cells
lacis cells
macula densa
columnar cells in juxtaglomerular apparatus
specialized part of distal convoluted tubule
nuclei tend to be more crowded together
juxtaglomerular cells
modified smooth muscle in wall of adjacent afferent arteriole
senses blood pressure via stretch receptors
secretes renin
mechanism of proteinuria
- dz in glomerulus
- increased quantity of proteins in serum (overflow proteinuria)
- Fanconi syndrome: low rabsorption at proximal tubule
why is proteinuria common in patients w/glomerelonephritis & diabetes?
b/c they lead to increased permeability of teh glomerular filter
proteinuria is the first indicator of…
renal dz
function of proximal convoluted tubule
reabsorb water, ions, nutrients, vitamins, & small plasma proteins
how can the proximal convoluted tubule & thick descending limb of the loop of Henle be differentiated?
both simple cuboidal epithelium
PCT has microvilli with glycocalyx on apical surface & margins between cells are indistinct b/c they have a well developed interlocking system of lateral cell processes for polarized transport
thick limb appears to have clear lumen
what cells secrete erythropoeitin?
fibroblastic interstitial cells of kidnery cortex
where is simple squamous epithelim found in the nephron?
thin descending limb of loop of Henle
loop of Henle
ascending limb of loop of Henle
what cell type forms the distal convoluted tubule?
small simple cuboidal cells w/sparse microvilli
how is net filtration pressure at the glomerular capillaries calculated when given cpillary & Bowman’s capsule hydrostatic & oncotic pressures?
Peffective = (PC - PBS) - (πC - πBS)
P= hydrostatic pressure
π= oncotic pressure
πBS= 0 b/c protein does not enter Bowman’s space
what properties determine if a substance is filtered thru the golmerulus?
size & charge
what size particles can be filtered by the glomerular filter?
<42 angstroms
<18 angstroms are freely filtered
18-42 angstroms are partially filtered & dependent on charge
how does charge effect glomerular filtration?
18-42 angstroms→ + filter at higher rate due to - charge of pores
<18 angstroms→ - filter at higher rate
nephrotic dz affects which cell type?
glomerular epithelial cells
aka podocytes
thus affecting composition of filtrate
nephritic dz affects which cell type?
capillary endothelial cell integrity & diameter
thus affecting magnitude of GFR
what is the total area of the glomerular capillaries?
3m2
what is the total pore area of the glomerular capillaries?
5%
0.15m2
what percentage of sodium is reabsorbed?
99.6%
what percentage of chloride is reabsorbed?
99.4%
what percentage of potassium reabsorbed?
92.7%
what percentage of bicarbonate is reabsorbed?
99.9%
what percentage of water is reaborbed?
99.1%
how does capillary oncotic pressure change over a systemic capillarty?
increases due to concentration of proteins as they do not pass thru the endothelium, but treated as 0
because systemic capillaries receive 5000L/day, but onlly filter 20L/day or 0.4%
how does capillary oncotic pressure change across a glomerular capillary?
increases
but not equal to 0 like systemic
because 20% of plasma is being filtered compared to the 0.4% seen in systemic circulation
how does πi and πBS compare?
similar; they both do not change
but πBS=0, because protein is not freely filtered in the glomerulus
how does PC compare in systemic v. glomerular systems?
systemic PC drops along length of capillary= driving force for reabsorption
glomerular PC is constant due to low resistance/wider radius (theoretically decreases but negligible amt)
what is PBS?
the hydrostatic pressure within the Bowman’s space
=0
b/c fluid flows thru tubular system before pressure is exerted
how does increased renal plasma flow influence GFR?
PC & PBS are constant
πBS is 0
πC decreased
therefore GFR increases
why does the πC curve less steeply with increased renal plasma flow?
concentration of proteins reduces more slowly relative to length of glomerular capillaries
→takes longer for πC=PC
→more fluid is filtered aka increased GFR
how does isolated increased afferent arteriolar resistance influence GFR?
decreases GFR proportionately via:
- decreased renal plasma flow
- decreases PC
how is flow calculated?
F=deltaP/R
flow= change in pressure/resistance
if efferent arteriolar resistance is higher than afferent arteriolar resistance, what effect is seen in GFR?
GFR is increased
how does isolated increased efferent arteriolar resistance affect GFR and why?
decreases GFR by reducing renal plasma flow
increases GFR by enhancing PC
overall: moderate increase in efferent arteriolar resistance increases GFR, while severe constriction reduces GFR
how does increased hydrostatic pressure in Bowman’s space influence GFR?
decreases GFR
(congestion in urinary system- stone in collecting duct?)
how does dehydration influence GFR?
dehydration increases glomerular capillary oncotic pressure
→decreases GFR
how does hunger influence GFR?
during fasting/starvation plasma proteins are decreased
→decreasing glomerular capillary oncotic pressure
→increasing GFR
where is the greatest pressure change found along renal vessels?
afferent arterioles esp. in HTN pts as the glomerular pressure is kept relatively constant
over what systemic blood pressure range can the RPF/GFR be held constant by autoregulation?
100-200 systolic
how is renal blood perfusion autoregulated?
- myogen mehanical stretch reflex receptors in smooth muscle of arterioles
- tubulo-glomerular feedback via juxtaglomerular apparatus
how does sympathetic nervous activity influence GFR?
minor→ only affects efferent arterioles→ no change in GFR
strong activity: dominant effect on afferent arterioles→ greatly reduce GFR (kidney shock)
how does atrial natriuretic peptide influence GFR?
dilates afferent arterioles
→increases RPF
→ increases GFR
how do prostaglandins influence GFR?
dilate arterioles
→ increase RPF
→ increase GFR
how does NO influence GFR?
dilates arterioles
→increases RPF
→increases GFR
how does the juxtaglomerular appartus regulate GFR?
- increased GFR
- increases NaCl delivery to macula densea in distal convoluted tubule
- enhanced NaCl uptake in macula densa via Na+/K+/2Cl- symporter
- increase ATP & adenosine (ADO) concentrations
- ATP binds P2X receptor & ADO binds A1 receptor on plasma membrane of smooth muscle of afferent glomerular arteriole
- increases intracellular Ca2+
- induces vasoconstriction
- returns GFR to normal
what inhibits renin secretion by granular cells in the afferent arteriole?
ATP & ADO induced increase in intracellular Ca2+
which is electrically coupled to
what happens NaCl delivery to the macula densa falls?
caused by reduced GFR
- ATP & ADO release decreases
- decreases intracellular Ca2+ in arteriolar smooth muscle
- stimulates granular cells to release renin
- macula densa also produces PGE2 that stimluates granular cell secretion of renin
effect of angiotensin II
enhances NaCl retention
which enhances the retention of water
what is the driving force for transcellular reabsorption?
tubular fluid starts out more negative than interstitial fluid
b/c small - ions filter faster than + ones
-3mV compared to interstitial 0mV & intracelluar -70mV
what is the driving force for the reabsorption of organic solutes in the first half of the proximal tubule?
sodium electrochemical gradient
maintained by basolateral Na+/K+ ATPase pump
organic solutes: glucose, lactate, amino acids, phosphate (all -)
what drives the reabsorption of water in the first half of the proximal tubule?
Na+, HCO3-, & organic solute reabosption→ increases interstitial osmolarity (293osM compared to 287osM in tubular fluid→ drving force for water aborption
water move both paracellularly & transcellularly
what transporters are found in the apical membrane in te first have of the proximal tubule?
Na+/organic solute symporter (glucose, phosphate, lactate, amino acids)
Na+/H+ antiporter
what transporters are found in the basolateral membrane of the first half of the proximal tubule?
Na+/K+ ATPase antiporter
K+ leak channel
organic solute transporters
HCO3- transporter
where are most organic solutes absorbed in the tubular system?
first half of the proximal tubule
in the first half of the proximal tubule, concentration of what ion increases in tubular fluid?
Cl-
HCO3- is freely filtered in the glomerulus, but how is it reaborbed?
usually as CO2 via passive diffusion into the cell
H+ pumped out of the cell reacts w/HCO3- in tubular fluid to form H2O & CO2
reacts with carbonic anhydrase w/in the cell to recycle H+ & HCO3- is transported into interstitium/blood
what transporters are found in the apical membrane in te second half of the proximal tubule?
Na+/H+ antiporter
anion-/Cl- antiporter
H & anion bind & enter cell via passive diffusion→ recycled
what transporters are found in the basolateral membrane in te second half of the proximal tubule?
Na/K ATPase antiporter
K/Cl symporter: against Cl electrogradient, but driven by K going down its electrochemical gradient
what is mainly reabsorbed in the second half of the proximal tubule?
NaCl
water follows
why is Cl- reabsorbed paracellularly in the second half of the proximal tubule?
because a gradient between tubular fluid & interstitium has been created
what is the consequence of paracellular Cl reabsorption in the second half of the proximal tubule?
tubular fluid becomes + compared to blood
causes paracellular Na reabsorption
water is reabsorbed as well
1/3 of NaCl reabsorption occurs this way
what solvents are dragged with paracellular water reabsorption?
K+, Ca++, Mg++
what happens to proteins that are filtered by the glomerulus?
very small amt/day
undergo endocytosis or are partially degraded by enzymes on the apical surface of the proximal tubule
broken down w/in cell
returned to blood as individual amino acids
**very easily saturated**
orgainc anions secreted in proximal tubule
cAMP, bile, hippurate, oxalate, prostaglandins, urate
organic cations secreted in proximal tubule
creatinine, dopamine, epinephrine, norepinephrine
how are organic anions & cations secreted into the proximal tubule?
non-specific transporters
what is the concentration of solutes in tubular fluid at the end of the proximal tubule v. plasma?
Na+ unchanged from ultrafilatrate
HCO3- decreased
Cl- increased
inulin increased by 3 fold
isotonic to plasma
what is reabsorbed in the thin descending limb of the loop of Henle?
water via aquaporin 1
no NaCl
what is the tonicity of tubular fluid compared to plasma at the end of the loop of Henle?
hypotonic
what transporters are found in the apical membrane in the thick ascending limb of the loop of Henle?
Na/K/2Cl symporter
K leak channel
Na/H antiporter
what transporters are found in the basolateral membrane in the thick ascending limb of the loop of Henle?
K/Cl symporter
Na/K ATPase antiporter
HCO3- transporter
why is the apical K+ channel in the thick ascending limb of the loop of Henle so important?
K+ is required for Na/K/2Cl symporter
also creates + charge in tubular fluid that drives paracellular absorption of Mg2+, Ca2+, K+, Na+
what segments of the tubular system are impermeable to water?
thick ascending limb of loop of Henle
early distal tubule
why is tubular fluid hypoosmolar at the end of the thick ascending limb of the loop of Henle?
because it reaborbs NaCl but is impermeable to water
what is absorbed in the early distal tubule?
NaCl
via apical symporter
basolaterally by Na/K ATPase antiporter & Cl- channel
where are principal cells located?
late distal tubule & collecting duct
where are intercalated cells found?
late distal tubule & collecting duct
what exchange occurs in principal cells & what is its driving force?
NaCl & water reabsorption & K secretions
Na absorbed by apical Na channel & basolateral Na/K ATPase antiporter (maintained by basolateral K channel)
K secretion occurs via apical K channel
Cl absorbed paracellularly driven by - luminal voltage created by Na reabsorption
water via aquaporin 2 on apical surface & 3 and 4 on basolateral surface
what reabsorption in teh late distal tubule and collecting duct is dependen on what?
presence of ADH (anti diuretic hormone)
what exchange occurs in intercalated cells & what is its driving force?
H+ secretion via ATPase pump in apical membrane
absorb K via K+/H+ ATPase antiport pump in apical membrane
HCO3- crosses basolateral membrane
how much water is reabsorbed in the late distal tubule & collecting duct?
variable 8-20% as this is the area of fine regulation
what type of hormone is aldosterone?
non-polar mineralcorticoid
thus it can passively diffusion thru the plasma membrane & has an intracellular receptor
what are the effects of aldosterone in the late distal tubule & collecting duct?
- increased expression of Na channels in apical membrane
- increased activity/permeability of Na channels in apical membrane
- increased amount of Na/K ATPase antiporters in basolateral membrane
- stimulates synthesis of ATP
overall enhancement of Na reabsorption→ increases paracellular Cl reabsorption & K secretion across apical membrane
what cells are influenced by aldosterone?
principal cells of the late distal tubule & collecting duct
where does angiotensin II influence NaCl reabsorption?
in the proximal tubule
how does aldosterone cause alkalosis?
increased Na+ reabsorption→ increases K+ secretion by principal cells→ increased H+ secretion by intercalated cells→ increased HCO3- in blood→ increases pH
what activates the RAA system?
RAA= renin-angiotensin-aldosterone
decrease in extracellular fluid volume
why does increased [K+]plasma instead of changes in [Na+]plasma trigger aldosterone release?
Nernst equation
because ECF/plasma levels of Na+ are high 140mM so small changes are unnoticeable
but ECF/plasma levels of K+ are normally 4mM so small movement of ions cause relatively large fluxuations
what is the function of 11beta hydroxy steroid dehydrogenase (HSD)?
breaks down cortisol in principal cells to cortisone (an inactive metabolite)
b/c cortisol is a glucocorticoid & glucocorticoid & mineralocorticoid receptors poorly distinguish between their substrates
thus w/o this enzyme the high levels of cortisol found in the blood would cause mineralocorticoid effects of Na+ retention
what inhibits HSD?
glycyrrhetinic acid in licorice
and carbenoxolone
what are the effects of hyperaldosteronism and why?
- Na+ retention
- hypervolemia
- hypertension
- edema
- hypokalemia
- muscle weakness
- constipation
- ECG changes
- alkalosis
- tetany, cramps
- less H+ bound to albumin→ free plasma Ca2+ binds albumin→ decreased [Ca2+]plasma → increased neuronal activation
- tetany, cramps
what effect does increased K+ excretion have on urine flow rate?
increases it
linear relationship
how does K+ reabsorption/secretion vary with K+ intake?
low K+ intake→ 3% reaabsorbed in distal tubule & 6% in collecting duct
normal-high K+ intake→ 10-100% secreted in distal tubule & 5-50% secreted in collecting duct
what is the normal range for plasma K+?
3.5-5.5mM
how is H+ excreted in tubular fluid?
usually as H2PO4- but limited by low [HPO42-] 40mmol/day
so also as NH4+ w/much higher capacity: ammonia (NH3) is from glutamine transamination to alpha-ketoglutarate
what effect does parathyroid hormone have on the kidneys?
increases Ca2+ reabsorption strongly in loop of Henle & distal tubule
what effect does vitamin D have on the kidneys?
aka calcitrol
increases Ca2+ reabsorption indistal tubule
what effect does calitonin have on the kidneys?
(from C cells of thyroid)
inhibits Ca2+ reabsorption in loop of Henle & distal tubule
where is the majority of Mg2+ reabsorbed?
thick ascending limb of the loop of Henle
where is Ca2+ reabsorbed in the tubular system?
65-70% in proximal tubule via apical Ca2+ channel & basolaterally by Ca2+/2H+ ATPase antiporter & 3Na+/2Ca2+ antiporter
29-34% in loop of Henle & distal tubule in parallel with Na+
how does plasma pH influence Ca2+ absorption?
alkalosis increases Ca2+ reabsorption in distal tubule
acidosis decreases Ca2+ reabsorption in distal tubule
how does plasma [PO43-] affect Ca2+ reabsorption?
induces PTH→ increases Ca2+ reabsorption
how does extracellular volume influence Ca2+ reabsorption?
reduced extracellular volume increases Ca2+ reabsorption
increased extracellular volume decreases Ca2+ reabsorption
how much fluid would be lost if urine is not concentrated?
if urine is isotonic (300mM) & 1200mmol/day of substances excreted→ 4L water lost
why is a it necessary to build a hypertonic interstitium?
to create a driving force for water reabsorption
and thus concentrate urine
how does the ascending limb of the loop of Henle produce high medullary interstitial OsM?
it pumps out salt, but is impermeable to water
therefore OsM of interstitium increases & tubular fluid decreases
how does the collecting duct contribute to concentrating of urine?
aquaporins in collecting duct allow for reabsorption of water from tubular fluid
driving force is OsM gradient produced in loop of Henle
what is the role of the vasa recta in concentrating urine?
to absorb & wash away excess water in the medullary interstitium
helps maintain hyperosmolar interstitium in countercurrent multiplier
how does tubular flow rate influence urine concentration?
slower tubular flow→ increased concentration
vice versa
linear relationship
how does the blood flow rate in the vasa recta affect urine concentration?
increase blood flow will absorb less interstitial water
decreasing OsM of interstitium
decreasing driving force for water reabsorption
decreasing urine concentration
how does ADH influence kidneys in concentrating urine?
controls water permeability in late distal tubule & collecting duct
can concentrate up to 400x
if present, water will be reabsorbed in late distal tubule & collecting duct→ concentrated/hyperosmolar urine
if not, opposite
what is pressure natriuresis?
the excretion of Na+ in response to increasing blood pressure→ leads to water loss→ reducing blood volume
most important mechanism for blood volume control
- mechanisms:
- increased bp→ increased blood flow to vasa recta→washes out medullary solutes→ reduces hypertonicity in medulla
- increase in arterial pressure→rapid decreases in # of apical Na+/H+ exchangers in proximal tubule
*
what is the effect of aldosterone on Na+ reabsorption?
increases NaCl reabsorption only, not water in thick ascending limb of the loop of Henle
increases NaCl & water reabsorption in distal tubule & collecting duct
what is the effect of angiotensin II on Na+ reabsorption?
increased NaCl & water reabsorption in the proximal tubule
what is the effect of arterial pressure on Na+ reabsorption?
increased atrial pressure decreases Na+ reabsorption
what is the effect of atrial natriuretic peptide on Na+ reabsorption?
decreases NaCl & water reabsorption in teh distal tubule & collecting duct
what is the effect of prostaglandins on Na+ reabsorption?
decrease the reabsorption of NaCl & water in the distal tubule & collecting duct
what is the effect of sympathetic nervous activity on Na+ reabsorption?
increases NaCl & water reabsorption throughout the nephron
stimulated by decreased ECF
how much can ADH affect GFR?
by 20%
how & where is urea exchanged in the tubular system?
reabsorbed via solvent drag in water in proximal tubule
secreted into tubular fluid by UT2 transports via facilitated diffusion on apical & basolateral membranes in the loop of Henle
reabsorbed in collecting duct by UT1 apically & UT4 basalaterally via facilitated diffusion
what is the relative permeability of urea throughout the nephron?
increased in medullary regions
majority in medullary collecting duct
also in medullary portion of loop of Henle
hydropenia
condition of restricted water intake
ex. kidney failure
what is the minimun urine flow?
0.3mL/min caused by extreme dehydration
what is the maximal urine flow?
16mL/min
exhibited by extreme water intake
how can water reabsorption be measured?
GFR/Vdot
how is ADH release controled?
- osmoreceptors sense increased interstitial osmolality
- usually caused by increased Na+ & - associates
- osmoreceptor stimluation provokes synthesis & release of ADH in hypothalamic-hypophysial system
- ADH enhances water reabsorption in late distal tubule & collecting duct
- dilution of ECF
where is the ADH receptor located?
basolateral plasma membrane of late distal tubule & collecting duct
what is the second messenger of ADH?
cAMP
ADH is also known as …..? why?
vasopressin
b/c it causes systemic vasoconstriction
how do osmoreceptors sense change in ECF environment?
they are very permeable to water soe they shrink when ECF is hypertonic & swell when ECF is hypotonic
what neurons communicate with osmoreceptors & baroreceptors to coordinate ADH release?
nucleus paraventricularis & nucleus supraopticus in the hypothalamus
signal posterior pituitary/hypophysis
what factors display a linear relationship with increasing ADH release?
increasing ECF osmolality above 280mOsm/kg H2O
&
decreasing blood volume starting at a 10% loss of blood volume (or change in blood pressure by 10%)
where is ADH stored?
neurohypophysis (aka posterior pituitary)
what dz state is caused by ADH deficiency?
diabetes insipidus
how does the thirst mechanism work?
intracellular dehydration causing an increase of OsM by more that 4mOsm/L
stimulates thirst center in hypothalamus
drinking water will dilutes ICF & reduce OsM to normal
what causes salt hunger?
decrease in Na+ in ECF
causes decrease in blood volume
stimulates hypothalamus
takes several hours or days
the absence of aldosterone is seen in what dz state?
Addison’s dz
edems is present when ECF volume exceeds?
25L
what relationship is normally seen between blood volume & ECF volume?
linear up to 7L of blood then plateau as increasing ECF creates edema
what is the most important mechanism for control of blood volume?
pressure diuresis
increase of Na+ excretion w/increased arterial pressure
what is the second messenger of ANP/BNP?
cGMP
where is BNP made?
ventricles of the brain
why is urodilatin more potent that ANP?
ANP=atrial natriuretic peptide
b/c some ANP in the kidneys is broken dow by endopeptidase that does not affect urodilatin
urodilatin
- secreted by: distal tubule & collecting duct
- stimulated by: rise in blood pressure & increased ECF volume
- function: inhibit NaCl & water reabsorption across medullary portion of collecting duct
what is an acid?
H+ dissociates from it
what is a base?
a H+ binds to it
what is a buffer?
an acid/base pair in which the pH does not vary greatly with the addition of loss of H+
Henderon-Hasselbalch equation
pH = pK + log ([A-]/[HA])
A=base
HA= acid
pK = log{ ([H+][A-]) / [HA] }
criteria for a good buffer
pKa within 1 pH unit
high enough concentration w/in system
what is the difference between closed & open system buffers?
in a closed system, [acid] + [base] must be constant
but in an open system, [acid] + [base] can be variable
how is buffering capacity measured?
beta (buffering capacity) = delta[base] / deltapH
steeper slope of pH v. base concentration⇒ high buffering capacity
flat = no buffering capacity
why is lactate a poor biological buffer?
high enough concentration in ECF, but pH is too low (pKa= 3.1) so it can only buffer pH 2.1-4.1 and body fluids are 6.8-7.4
why is phosphate a poor biological buffer?
pKa= 6.8 thus can buffer pH 5.8-7.8
but not found in high enough concentrations in ECF (only 2mM)
why is bicarbonate a good biological buffer when it’s pKa is 6.1?
0.9-1.3 pH units off of biological 6.8-7.4
is found in adequate concentrations (27mM ECF/10mM ICF)
**b/c it is the only buffer system that is OPEN in the body via the lungs as CO2 leaves it changes the equilbrium**
which amino acid are good buffers at normal biological pH?
His, Cys, & terminal NH2
why are proteins not a good biological buffer?
with a mix of amino acids, nearly any pH can be buffered by a protein
but they are relatively absent from ECF & plasma
