Chapter 6: Diseases of the Immune System Part 2 Flashcards
What are the 3 immune-privileged sites?
- Brain
- Eyes
- Testis
How are antigens restricted to peripheral tissues able to be expressed in the thymus during the process of central tolerance?
A protein called AIRE
Mutations in the AIRE gene cause?
Autoimmune polyendocrinopathy
Function of CTLA-4 and PD-1?
- Structurally homologous to CD28 and act as inhibitory receptors
- CTLA-4 has a higher affinity for B7 molecules on APC’s
- Act to induce anergy in a lymphocyte upon recognizing a self antigen
What plays a role in acceptance/tolerance of a fetus while inside the placenta?
CD4+ CD25+ FOXP3 Treg cells
Which cytokine is essential for the maintenance of Treg cells?
IL-2
How are self-reactive T cells able to undergo apoptosis in the process of peripheral tolerance?
- Self-reactive T cells express pro-apoptotic member of Bcl family (Bim), w/o antiapoptotic members of the family like Bcl-2 or Bcl-x
- If self-antigens engage antigen receptors of self-reactive T cells, FasL and Fas are co-expressed inducing extrinsic pathways of apoptosis
Mutations in the FAS gene cause what disease?
Autoimmune Lymphoproliferative Syndrome (ALPS)
What HLA type is associated with Ankylosing spondylitis?
B-27 (mainly B*2705 and B*2702)
Which gene is said to be most frequently implicated in autoimmunity; encodes what?
Associated with what disorders?
- PTPN22; encodes a protein tyrosine phosphatase
- Type I DM, Rheumatoid arthritis, IBD
Polymorphisms in the gene for NOD2 are associated with what disease?
Chron’s disease
Polymorphisms in the genes encoding the IL-2R (CD25) and IL-7 receptor α chains are associated with what 2 diseases?
MS
Type I diabetes
What are 2 methods by which infections may induce autoimmunity?
1) Infections may upregulate the expression of co-stimulators on APCs; if these cells are presenting self-antigens result may be a breakdown of anergy and activation of self-reactive T cells
2) Molecular mimicry: microbe expresses Ag w/ same AA sequence as self-Ags
What is the classic example of molecular mimicry?
Causes what?
- Rheumatic heart disease; Abs against streptococcal proteins cross-react w/ myocardial proteins
- Cause Myocarditis
Some viruses like EBV and HIV cause __________which may result in the production of autoantibodies
Polyclonal B cell activation
How may infections actually protect against some autoimmune diseases?
Promoting low levels of IL-2 production which is essential for maintaining Treg cells
Most chronic inflammatory diseases are caused by abnormal and excessive?
TH1 and TH17 responses
*Psoriasis, MS, and some types of IBD
What group of people is most common to develop SLE?
- Women
- African American and Hispanic > White
What ANAs are specific for SLE?
- anti-dsDNA
- anti-Smith (Sm)
What antibodies are diagnostic for Sjogren syndrome?
- anti-Ro/SS-A
- anti-La/SS-B
Autoantibodies associated with Goodpasture Syndrome?
- anti-basement membrane
- anti-type IV collagen
Autoantibodies associated with drug-induced SLE?
anti-histone antibodies
Autoantibody specific for Rheumatoid Arthritis?
anti-CCP (cyclic citrullinated peptides)
Autoantibody associated with diffuse systemic scleroderma?
Limited systemic scleroderma?
- Diffuse = anti-DNA topoisomerase (anti-Scl 70)
- Limited (CREST) = anticentromere antibody
Injury in SLE is caused by?
What type of hypersensitivity?
- Deposition of immune complexes and binding of antibodies to various cells and tissues
- Type III hypersensitivity
Hallmark of SLE?
Production of autoantibodies
Patients with SLE that have the presence of antiphosphlipid antibodies will have increased?
These antibodies cause what kind of state?
- Increased PTT
- Hypercoagulable state (excessive clotting); leading to thrombosis
Specific alleles of which HLA has been linked to production of anti-dsDNA, anti-Sm, and anti-phospholipid antibodies seen in SLE?
HLA-DQ
What environmental factors are involved in SLE?
- UV light: may induce apoptosis in cells and alter DNA to become immunogenic
- Gender bias: partly related to genes on the X chromosome
- Drugs: such as hydralazine, procainamide, and D-penicillamine
What is the morphology of the blood vessels like in patients with SLE?
How about in the chronic stages?
- Acute necrotizing vasculitis involving capillaries, small arteries, and arterioles
- Chronic stages = vessels undergo fibrous thickening with luminal narrowing
What are the joints like in patients with SLE?
Opposite of what?
- Non-erosive synovitis with little deformity
- Opposite of RA
Glomerular lesions in SLE are the result of what?
Immune Complex Deposition
What is the most common and also the most severe pattern of glomerular disease seen in SLE?
Characteristics/morphology?
- Diffuse lupus nephritis (Class IV)
- >50% involvement of Glomeruli
- Proliferation of epithelial cells —> cellular crescents that fill Bowmans space
- Circumferential thickening of capillary wall, forming “wire loop” strucutres on light mircoscopy (see attached photo)

Venous and arterial thromboses, which may be associated with recurrent spontaneous miscarriages and focal cerebral or ocular ischemia is characteristic of?
Antiphospholipid antibody syndrome
- Can be primary = by itself
- Or secondary = in association with lupus
Immunofluorescence microscopy of the skin in patient with SLE will show?
Are these finding diagnostic?
- Deposition of immunoglobulin and complement along dermoepidermal junction
- May also be present in uninvolved skin
- This finding is NOT diagnostic of SLE and is sometimes seen in scleroderma or dermatomyositis

What kind of effusions may be present with SLE?
Pleural and pericardial effusions (sometimes with LE cells)
What cardiovascular system effects are present with SLE?
- Symptomatic or asymptomatic pericardial involvement (50% of pt’s)
- Myocarditis
- Valvular abnormalities (mitral and aortic)
- Valvular (Libman-Sack) endocarditis (see photo)
- CAD (angina, MI) owing to coronary atherosclerosis

What other organs are involved in SLE?
CNS
Spleen (onion-skin lesions from smooth muscle cell hyperplasia)
Lungs (effusions), interstitial fibrosis and secondary pulmonary HTN
What are signs of renal involvement in SLE?
Hematuria, proteinuria, red cell casts, nephrotic syndrome
What are the main clinical features of a patient presenting with SLE?
- Butterfly rash over the face
- Fever
- Pain but no deformity in one or more peripheral joints (feet, ankles, knees, hips, fingers, wrists, elbows, shoulders)
- Pleuritic chest pain
- Photosensitivity
What are the most common causes of death in patients with SLE?
- Renal failure
- Infections
- CAD
How does Chronic Discoid Lupus Erythematosus present?
- Skin manifestations mimicking SLE, rarely systemic involvement
- Skin plaques w/ edema, erythema, scaliness, follicular plugging
- Localized, deep, and scarring
- Usually only involving the face and scalp
How is Subacute Cutaneous Lupus distinguished from Chronic Discoid LE?
- Skin rash is widespread, superficial, and non-scarring
- Most have mild systemic symptoms consistent with SLE
- Strong association with antibodies to the SS-A antigen and w/ HLA-DR3 genotype
*Basically an intermeditate between SLE and Chronic Discoid LE
What drugs induce lupus syndrome?
Hydralazine
Procainamide
Isoniazid
D-penicillamine
*Anti-TNF used to tx RA, may also induce lupus*
What organs are NOT affected in drug-induced LE?
Renal
CNS
What is Sjorgren characterized by?
Result of?
- Dry eyes (keratoconjunctivitis sicca) and dry mouth (xerostomia)
- Immunologically mediated destruction of lacrimal and salivary glands
Sjorgen Syndrome can occurs as a primary form (sicca syndrome) or most commonly as a secondary form, which disease is it most commonly associated with?
- Rheumatoid arthritis
- Can also be associated with SLE, polymyositis, scleroderma, vasculitis, mixed CT disease, or thyroiditis
Which autoantibody found in patients with Sjorgen syndrome is associated with early disease onset, longer disease duration, and extraglandular manifestations, such as diffuse pulmonary fibrosis, cutaneous vasculitis and nephritis?
SS-A
What is the earliest histological finding in both the major and minor salivary glands in patients with Sjorgen Syndrome?
Periductal and perivascular lymphocytic infiltration
Patients with Sjorgen Syndrome are at a high risk for developing?
Dominant B-cell clone and marginal zone lymphoma
What are the clinical features and presenting signs of a patient presenting with Sjorgen Syndrome?
Most common gender and age group affected?
- Women between ages 50-60
- Keratoconjunctivitis = Blurring of vision, burning, and itching, and thick secretions accumulate in the conjunctival sac
- Xerostomia = Difficulty swallowing solids, decreased taste, cracks and fissures in mouth
- Parotid gland enlargement
- Epistaxis
- Recurrent bronchitis and pneumonia
What is essential for the diagnosis of Sjorgen Syndrome?
Biopsy of the lip to examine minor salivary glands
What sort of renal involvement is seen in Sjorgen Syndrome vs. SLE?
- Defects of tubular function (renal tubular acidosis, uricosuria, and phosphaturia)
- Glomerular lesions are extremely rare (like those seen in SLE)
Systemic Sclerosis (Scleroderma) is characterized by what 3 things?
1) Chronic inflammation though to be result of autoimmunity
2) Widespread damage to small blood vessels
3) Progressive interstitial and perivascular fibrosis in the skin (early) and multiple organs (later)
Systemic Sclerosis (Scleroderma) is characterized by excessive?
Fibrosis throughout the body
Which organs are most commonly involved in Systemic Sclerosis (Scleroderma)?
- Skin is most common
- GI tract
- Kidneys
- Heart
- Muscles
- Lungs
Majority of patients with Systemic Sclerosis (Scleroderma) die how?
- Renal failure
- Cardiac failure
- Pulmonary insufficiency
- Intestinal malabsorption
Some patients with the limited form of Systemic Sclerosis (Scleroderma) also develop?
- CREST syndrome
- Calcinosis
- Raynaud phenomenon
- Esophageal dysmotility
- Sclerodactyly
- Telangiectasia
What are the Clinical features of Systemic Sclerosis (Scleroderma)?
Who’s affected most often (gender and age)?
- Female-to-male ratio of 3:1
- More severe in blacks
- Peak incidence in the 50-60 yo group
- Distinctive features = cutaneous changes, skin thickening, Raynaud phenomenon
- Dysphagia, respiratory difficulty, myocardial fibrosis, mild proteinuria
What is the major cause of death in patients with Systemic Sclerosis (Scleroderma)?
Pulmonary disease
What leads to the fibrosis in Systemic Scleroderma?
Activation of fibroblasts by cytokines produced by T cells and alternatively activated macrophages (M2)
Mixed Connective Tissue disease is characterized serologically by high titers if antibodies to?
Ribonucleotide particle-containing U1 ribonucleoprotein (anti-U1-ribonucleotide)
How does Mixed CT disease typically present?
Good response to what tx?
- Synovitis of the finger
- Raynaud phenomenon and mild myositis
- Renal involvement is modest
- Good response to corticosterioid, in the short term
What disease often affects middle-aged and older men and is 1st characterized in autoimmune pancreatitis?
IgG4 Related Disease
What is Mikulicz syndrome?
Seen in what conditions?
- Enlargement and fibrosis of salivary and lacrimal glands
- Sjorgen syndrome and IgG4 related disease
What is IgG4 related disease characterized by?
- Tissue infiltrates dominated by IgG4 anti-body producing plasma cells and lymphocytes (mainly T cells)
- Storiform fibrosis
- Obliterative phlebitis
- Increased serum IgG4
What is the direct pathway for recognition of alloantigens in organ grafts?
Why are the DCs in the donor organs most important for initiating the antigraft response?
- T cells of recipient recognize allogenic (donor) MHC molecules on the surface of donor APCs in the graft
- The APCs of the donor organ express high levels of class I and class II MHC molecules and are also endowed with costimulatory molecules (B7)

What is involved in the indirect pathway of allorecognition in organ grafts?
Which immune cells are involved here?
- Peptides derived from the donor tissue are presented by the host’s own MHC molecules, like any other foreign peptide
- CD4+ T cells enter the graft and recognize graft antigens being displayed by host APCs that have also entered the graft, resulting in delayed hypersensitivity type of inflammation.
- CD4+ T cells also stimulate B lymphocytes —> plasma cells to produce antibodies and also macrophages
- CD8+ CTLs may be generated via this pathways, but cannot kill graft cells, because these CTLs recognize graft antigens presented by host APCs and cannot recognize the graft cells directly

What kind of damage occurs from CD8+ CTLs that may be generated from the indirect pathway for recognition of alloantigens in organ grafts?
What is the principle mechanism of cellular rejection in the indirect pathway?
- CD8+ CTLs CANNOT kill graft cells becauses these CTLs recognize graft antigens presented by the host APCs and cannot recognize the graft cells directly
- Principle mechanism of cellular injury = T-cell cytokine production and inflammation
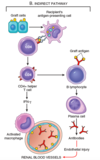
What characterizes acute cellular rejection; cell type responsible?
Graft injury is caused by?
Commonly seen when?
- CD4+ T cells secrete cytokines causing inflammation characterized by increased vascular permeability and local accumulation of mononuclear cells (lymphocytes and macrophages)
- Graft injury is caused by activated macrophages
- Occurs within initial months following transplantation

What characterizes chronic cellular rejection of a transplanted organ?
Patients may present clinically with progressive renal failure manifested by a rise in serum levels of?
- Lymphocytes reacting against alloantigens in the vessel wall secrete cytokines that induce local inflammation
- May stimulate proliferation of vascular endothelial and smooth muscle cells
- Rise in serum creatinine over period of 4-6 months
What causes hyperacute rejection of an donor organ?
Most commonly seen in what circumstances?
How fast is the rejection?
- Occurs when preformed antidonor antibodies are present in circulation of reciepient
- Can be caused by: previous transplant, prior blood transfusions, and multiparous women
- Occurs within minutes to hours

Fibrinoid necrosis ans thrombosis are typical of what kind of rejection reaction?
Hyperacute rejection

In acute antibody-mediated rejection what seems to be the target of the antibodies formed?
How quick does this rejection reaction occur?
- Graft vasculature
- May occur within days or may suddenely appear months or even years later after immunosuppression is tapered or terminated












