ORGANIC 1: Chapter 1: Hybridization & Bonding Flashcards
What is the hybridization of the indicated atom?

sp2
What is the hybridization of the indicated atom?

sp3
What is the hybridization of the indicated atom?

sp3
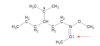
What is the hybridization of the indicated atom?

sp
What is the hybridization of the indicated atom?

sp
What is the hybridization of the indicated atom?

sp3
What is the hybridization of the indicated atom?

sp3
What is the hybridization of the indicated atom?

sp2
What is the hybridization of the indicated atom?

sp3
What is the hybridization of the indicated atom?

sp2
What is the hybridization of the indicated atom?

sp3
What is the relationship between bond length & bond strength?
The shorter the bond, the stronger it is and
the longer the bond, the weaker it is
Which of the indicated bonds in the molecule shown below is the longest?

A
Which of the indicated bonds in the molecule shown below is the shortest?

C
Which of the indicated bonds in the molecule shown below is the strongest?

C
Which of the indicated bonds in the molecule shown below is the weakest?

A
Sigma bonds are formed from the overlap of _______ orbitals.
Hybrid orbitals (sp, sp2, sp3, etc.) or atomic orbitals (hydrogen-hydrogen/carbon-hydrogen)
Pi bonds are formed from the overlap of _______ orbitals.
P orbitals
What type of bonding is depicted below?

Sigma bonding
What type of bonding is depectied below?

Sigma anti-bonding
What type of bonding is depectied below?

Pi bonding
What type of bonding is depectied below?

Pi anti-bonding
What is the formula to calculate formal charge?
Formal Charge = # valence electrons - # bonds - # electrons
or
Formal Charge = # valence electrons - # lines - # dots
What is the formal charge of the indicated atom?

0
What is the formal charge of the indicated atom?

+1