10. Metabolism and enzymes Flashcards
Anabolic and catabolic reactions in the cell are controlled in the presence of specific what?
Enzymes.
There are thousands of chemical reactions occurring constantly within all living cells. Together, these are a cell’s what?
Metabolism.
This varies from cell to cell, but it is important to remember that these are not random reactions; each one occurs specifically, at a certain location, speed, and time, to allow the cell to get the energy it needs to function and survive.
A chain of reactions is called a pathway, and each step of a pathway is controlled by a what?
Enzyme.
The first type of reaction is what?
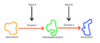
Catabolic.
These are reactions that break down large molecules into smaller, simpler molecules.
An everyday example of a catabolic reaction is when starch breaks down into what?
Glucose. This is catabolic reaction.
In catabolic reactions, large molecules are broken down into smaller molecules.
When these reactions occur, a net amount of energy is released. This is called a what reaction?
Exergonic reaction (think ex = exit = out).
The other main type of reaction is called an anabolic reaction. This is where what?
Smaller molecules are brought together to build larger molecules.
An example of an anabolic reaction is what?
An example of this is forming proteins. Small amino acid molecules are bonded together to form larger proteins.
It takes energy to build these bonds, and these are called what reactions?
Endergonic reactions, as they absorb energy. A net amount of energy is then stored in these bonds, so the product has more energy than the reactants.
Energy is carried between reactions by what?
ATP molecules (adenosine triphosphate).
In any reaction, what type of energy required for the reaction to occur?
Activation energy.
What do enzymes do to the activation energy level required?
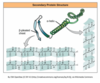
Enzymes lower the activation energy so that the reactions can happen much faster. They provide an alternative pathway, where the substrate binds to the active site of the enzyme.

Enzymes are large what type of molecule?
Globular protein molecules.
The long amino acid chains fold into a specific 3D shapes. This is described as what type of protein structure?
Tertiary.
Enzymes are very specific. They will catalyse one or two very specific reactions.
This is because enzymes have an active site that is specific for the molecules in that reaction. The substance that the enzyme acts upon is called the what?
Substrate.
Biochemical processes are controlled and regulated by a series of specific enzymes. For example the reactions in glycolysis, which is the first reactions in respiration, requires a series of how many steps, each with its own specific enzyme?
Nine steps.
There are two main models of enzyme specificity.
The first is what?
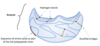
The Lock and Key Model.
The enzyme has an active site which specifically fits the substrate, like a key fitting a lock. If the substrate doesn’t fit in the active site, then the reaction will not occur.