CORE Stroke and Vascular Anatomy Flashcards
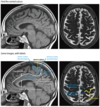
Central Sulcus
The central sulcus separates the ______ strip (______ lobe) from the _______ cortex (_______ lobe).
How do you find the central sulcus on the sagittal plane?
How do you find central sulcus on the axial plane?
- The central sulcus separates the motor strip (frontal lobe) from the sensory cortex (parietal lobe).
- To find the central sulcus, follow the cingulate sulcus posteriorly on a slightly off midline sagittal. The cingulate sulcus connects to the marginal ramus. Directly anterior to the marginal ramus is the paracentral lobule, which contains both the motor strip and the sensory cortex.
- On an axial image, the central sulcus forms a characteristic upside down omega. The corresponding region of the motor strip, just anterior to the, controls the hand.
What are the segments of the Internal Carotid Artery?
Include important branches off of the ICA and off what specific segment
- Cervical (C1): does not branch within the neck.
- Petrous (C2): Fixed to bone as the ICA enters the skull base, so a cervical carotid dissection is unlikely to extend intracranially.
- Lacerum (C3): No branches.
- Cavernous (C4): The meningohypophyseal trunk arises from the cavernous carotid to supply the pituitary, tentorium, and dura of the clivus. The inferolateral trunk also arises from C4 to supply the 3rd, 4th, and 6th cranial nerves, as well as the trigeminal ganglion.
- Clinoid segment (C5): The carotid rings are two dural rings that mark the proximal and distal portions of the clinoid segment of the ICA. The carotid rings prevent an inferiorly located aneurysm from causing intracranial subarachnoid hemorrhage with rupture.
-
Supraclinoid (C6 Ophthalmic -C7 Communicating): gives off several key arteries:
- The ophthalmic artery supplies the optic nerve. It takes off just distal to the distal carotid ring in 90% of cases and can be used as a landmark for the distal ring. Aneurysms located superior to this ring can result in subarachnoid hemorrhage. Given this risk, these aneurysms are treated more aggressively than aneurysms located proximal to the distal dural ring, which are contained.
- The posterior communicating artery (P-comm) is an anastomosis to the posterior circulation. A fetal posterior cerebral artery (PCA) is a variant supplied entirely by the ipsilateral ICA via an enlarged P-comm.
- The anterior choroidal artery supplies several critical structures, despite its small size. It supplies the optic chiasm, hippocampus, and posterior limb of the internal capsule.
- MNEMONIC: CPLCCOC

What are the persistent fetal connections of the anterior and posterior circulation?
Include origin, what it feeds, and location of each.
Which one is most common? Clinical significance? Angiographic appearance? What are the two types of this most common one?
-
Trigeminal: cavernous carotid, top of basilar, suprasellar cistern
- A persistent trigeminal artery is the most common persistent carotid-basilar connection and has an association with aneurysms.
- The persistent trigeminal artery courses adjacent to the trigeminal nerve. Angiography shows a characteristic trident or tau sign on the lateral view due to the artery’s branching structure.
- Saltzman type I connects to the basilar artery while Saltzman type II connects to the superior cerebellar artery.
- Otic: petrous carotid, mid-basilar, internal auditory canal
- Hypoglossal: high cervical internal carotid at the skull base, intracranial vertebrobasilar circulation, hypoglossal canal
- Proatlantal type 1: low internal carotid, cranial and cervical vertebrobasilar circulation, C2 level.
- Proatlantal type 2: external carotid, cranial and cervical vertebrobasilar circulation, C2 level.MNEMONIC: PPHOT (this is upside down from above but goes along with mnemonic for segments of ICA - CPLCCOC)

Circle of Willis (COW)
Name it all baby
What are all the critical small arteries arising from the COW? Provide origin and the structures it feeds.
- The anterior choroidal artery is the most distal branch of the internal carotid artery and supplies the optic chiasm, hippocampus, and posterior limb of the internal capsule.
- The A1 segment of the anterior cerebral artery travels above the optic nerves and give off the recurrent artery of Heubner, which supplies the caudate head and anterior limb of the internal capsule. The A1 segment also gives rise to the medial lenticulostriate perforator vessels, which supply the medial basal ganglia.
- Just outside the circle of Willis, the middle cerebral artery gives rise to the lateral lenticulostriate perforator vessels to supply the lateral basal ganglia including the lateral putamen, external capsule, and the posterior limb of the internal capsule.
- The posterior communicating artery travels between the optic tract and the 3rd cranial nerve, giving off anterior thalamoperforator vessels. A P-comm aneurysm may cause cranial nerve III palsy due to local mass effect.
- The posterior cerebral artery gives off thalamoperforators to supply the thalamus. Artery of Percheron is a variant where there is a dominant thalamic perforator supplying the ventromedial thalami bilaterally and the rostral midbrain, arising from a P1 PCA segment. An artery of Percheron infarct will result in bilateral ventromedial thalamic infarction, with or without midbrain infarction (the infarct may be v-shaped if the midbrain is involved). Deep venous thrombosis may also result in bilateral thalamic infarcts.
- MNEMONIC - recurrent artery of Huebner comes off A1 while artery of Percheron comes off of the PCA.
- Medial lenticulostriates perforator vessels come off ACA while lateral lenticulostriate vessles come off MCA (makes sense anatomically).

What are the Circle of Willis anatomic variants?
Normal COW anatomy is seen in approximately what percent of the time?
In what scenario can you have an isolated ICA?
- Normal circle of Willis anatomy is only seen approximately 25% of the time.
- Hypoplastic (34%) or absent (rare) PCOM.
- Fetal PCA (~20%): PCA supplied by ICA
- Hypoplastic P1: pretty similar to fetal PCA
- Hypoplastic (10%) or absent (rare) A1
- Absent ACOM (5%)
- Azygous ACA (3%): associated with holoprosencephaly
- Double of Plexiform ACOM: associated with aneurysms.
- Can have a fetal origin of PCA and absent A1 = anatomically isolated ICA = restricted potential collateral blood flow!

Vascular Arterial Territories
Name it all baby!

ACA Distribution
- Anterior cerebral artery (ACA) distribution. Shaded areas of these axial diagrams, arranged in sequence from base to vertex, outline the territory of the ACA including the medial lenticulostriate (orange), callosal (blue), and hemispheric branches (green).

MCA Distribution
- Middle cerebral artery (MCA) distribution. This diagram of the axial sections, arranged in sequence from base to vertex, outlines the MCA distribution with the lateral lenticulostriate (orange) and hemispheric branches (green).

PCA Distribution
- Posterior cerebral artery (PCA) distribution. Axial diagrams arranged in sequence from base to vertex outline supply from the PCA, the thalamic and midbrain perforators (orange), callosal (blue), and hemispheric branches (green).

Segmental Anatomy of the MCA
Describe extent each segment.
Where is the transition from the M1 to the M2 segment?
- Although the transition from M1 to M2 is technically defined as the upward point of deflection into the Sylvian fissure, in practical terms, the pre-bifurcation MCA is often called M1 and the post-bifurcation MCA is called M2.

Segmental Anatomy of the ACA
What are the segments and branches of the ACA?
Where does the recurrent artery of Huebner arise from? What does it supply?
- The recurrent artery of Heubner arises most commonly from the A1 segment of the ACA, proximal to the anterior communicating artery. The recurrent artery of Heubner supplies the head of the caudate and the anterior limb of the internal capsule.

Imaging of Stroke
- The goal of stroke _______ is to determine who would benefit from therapy.
- The goal of stroke ________ is to restore perfusion to the brain.
- Discuss the American Heart Association guidelines for early management of adults with ischemic stroke (2018).
- The goal of stroke imaging is to determine who would benefit from therapy.
- The goal of stroke therapy is to restore perfusion to the brain.
- Administer IV tPA within 3 hrs of last known normal (up to 4.5 hrs in some patients)
- NCCT and glucose check prior to IV tPA
- For patients who may be candidates for mechanical thrombectomy a CT angio or MRA is done.
- Patients ≥18 years should undergo mechanical thrombectomy with a stent retriever if they have minimal prestroke disability, have a causative occlusion of the internal carotid artery or proximal middle cerebral artery, have a National Institutes of Health stroke scale score of ≥6, have a reassuring noncontrast head CT (ASPECT score of ≥6), and if they can be treated within 6 hours of last known normal.
- In selected acute stroke patients within 6-24 hours of last known normal who have evidence of a large vessel occlusion in the anterior circulation -> get diffusion imaging to see if the patient is a candidate for mechanical thrombectomy.
- In selected acute stroke patients within 6-16 hours of last known normal who have a large vessel occlusion in the anterior circulation and meet other DAWN or DEFUSE 3 eligibility criteria, mechanical thrombectomy is recommended (6-24 hours is reasonable).
- Administration of aspirin is recommended in acute stroke patients within 24-48 hours after stroke onset.
- Some institutions add additional exclusion criteria for administration of intravenous tPA, although these additional criteria are not a part of the AHA guidelines. What are the three additional exclusion criteria?
- Individuals with a large (greater than 1/3 MCA territory) infarct may be excluded from IV tPA.
- Occlusion of the distal internal carotid artery and proximal MCA and ACA (a T-shaped occlusion) may preclude treatment with Iv tPA.
- Absence of a penumbra of salvageable brain that represents at least 20% of the region of abnormal perfusion may preclude treatment with Iv tPA.
Perfusion Stroke Imaging
What is the goal of perfusion imaging?
What in the world is a penumbra?
The penumbra has how much perfusion (mL/100g tissue per minute) vs how much in the normal gray matter?
What is the infarct core?
- The goal of perfusion imaging is to characterize the ischemic penumbra, which is the area of vulnerable brain adjacent to the infarct core that may also become infarcted without intervention.
- The penumbra does receive some perfusion, but at a reduced rate compared to normal brain.
- Perfusion of the penumbra is <20 mL/100 g tissue per minute in physiologic studies, compared to >60 mL/100 g tissue per minute for normal gray matter. Such a low rate of perfusion causes cellular dysfunction and produces a neurologic deficit, which may be restored with therapy.
- The infarct core is usually dead tissue, which generally cannot recover even after therapy.
NCCT Imaging of Acute Stroke
Why do we do NCCT to eval hyperacute infarcts? Whats the main purpose?
The sensitivity of NCCT for stroke?
What imaging findings can you see on NCCT in hyperacute stroke?
What window should one evaluate hyperacute stroke on NCCT?
- Noncontrast CT is the initial test of choice for evaluation of hyperacute infarct when the patient presents within the Iv tPA time window (3 hours, or 4.5 hours at some institutions).
- The main purpose of a noncontrast CT is to exclude patients who would be harmed by thrombolytic therapy, most importantly to exclude those with hemorrhage.
- Noncontrast CT in the hyperacute stage is relatively insensitive to detect early infarction compared to MRI. Subtle loss of gray-white differentiation in the insula or basal ganglia may be present on CT, thought to be due to decreased cerebral blood volume.
- The insular ribbon sign describes the loss of gray-white differentiation in the insula. The gray-white junction becomes most conspicuous at very narrow stroke windows (window 30/level 30).
- Obscuration of the lentiform nucleus (putamen and globus pallidus) is caused by loss of gray-white differentiation at the border of the lentiform nucleus and the posterior limb of the internal capsule.
- The hyperdense artery sign describes the direct visualization of the acute intravascular thrombus, most commonly seen in the MCA. The hyperdense artery sign is specific for ischemia when seen, but relatively insensitive (seen in approximately one-third of cases). Some authors suggest that the presence of the hyperdense artery sign portends a worse prognosis.




