C1 Oil, alkanes and fuels Flashcards
What is crude oil?
Mixture of hydrocarbons
What is a mixture?
2 or more substances mixed but not chemically bonded together
What is the general formula for alkanes?
CnH2n+2
What is a hydrocarbon?
Compound made from only carbon and hydrogen
Are alkanes saturated or unsaturated?
Saturated
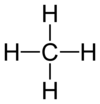
Draw a methane molecule.
Draw an ethane molecule.

Give the formula for a propane molecule.
C3H8
Name C4H10.
Butane
What does the line in between C-C in a molecule show?
Covalent bond, a shared pair of electrons
What method is used to separate crude oil?
Fractional distillation
Describe how fractional distillation works?
Crude oil is heated to evaporate and then the different fractions are condensed because of their different boiling points
As the number of carbons increase in an alkane what happens to the boiling point?
It increases
As the number of carbons increase in an alkane what happens to the viscosity?
It increases, gets more sticky
As the number of carbons increase in an alkane what happens to the flammability?
Gets less flammable

