2 - 7 - BIOLOGY OF HAIR FOLLICLES Flashcards
(16 cards)
Hair cycle and anatomy.
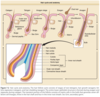
The upper follicle consists of the infundibulum and the isthmus, and the lower follicle consists of the suprabulbar and the bulbar areas (Fig. 7-2). 67,68 The upper follicle is permanent, but the lower follicle regenerates with each hair follicle cycle. The major compartments of the hair from outermost to innermost include the connective tissue sheath, the outer root sheath, the inner root sheath, the cuticle, the hair shaft cortex, and the hair shaft medulla, each characterized by distinct expression of the hair follicle–specific keratins (Table 7-2).

DERMAL PAPILLA

The dermal papilla (see Fig. 7-1) is a core of mesenchymally derived tissue enveloped by the matrix epithelium. It is comprised of fibroblasts, collagen bundles, a mucopolysaccharide-rich stroma, nerve fibers, and a single capillary loop. It is continuous with the perifollicular sheath (dermal sheath) of connective tissue that envelops the lower follicle.
Tissue recombination experiments have shown that the dermal papilla has powerful inductive properties, including the ability to induce hair follicle formation when transplanted below non–hair-bearing footpad epidermis. 77,78 This shows that the tissue patterning established during the fetal period can be altered under appropriate conditions. In human follicles, the volume of the dermal papilla correlates with the number of matrix cells and the resulting size of the hair shaft.79 In mice, the sizes of the hair bulb and hair diameter strongly depend of the proliferative activity of the matrix keratinocytes.80
Many soluble growth factors that appear to act in a paracrine manner on the overlying epithelial matrix cells originate from the dermal papilla. Specifically, keratinocyte growth factor (KGF) is produced by the anagen dermal papilla, and its receptor, FGF receptor 2 (FGFR2), is found predominantly in the matrix keratinocytes. Injections of KGF into nude mice produce striking hair growth at the site of injection, 81 suggesting that KGF is perhaps necessary for hair growth and cycling. However, surprisingly, KGF knockout mice develop morphologically normal hair follicles that produce “rough” or “greasy” hair; thus, KGF’s effects on hair follicle morphogenesis and cycling appear dispensable or replaceable by other growth factors with redundant functions.82
HAIR FOLLICLE INNERVATION
Myelinated sensory nerve fibers run parallel to hair follicles, surrounding them and forming a network (reviewed in83 ). Smaller nerve fibers form an outer circular layer, which is concentrated around the bulge of terminal follicles and the bulb of vellus follicles. Several different types of nerve endings, including free nerve endings, lanceolate nerve endings, Merkel cells, and pilo-Ruffini corpuscles are found associated with hair follicles. 84 Each nerve ending detects different forces and stimuli. Free nerve endings transmit pain, lanceolate nerve endings detect acceleration, Merkel cells sense pressure, and pilo-Ruffini structures detect tension. Perifollicular nerves contain neuromediators and neuropeptides, such as substance P and calcitonin gene-related peptide, that influence follicular keratinocytes and alter hair follicle cycling. 41,85-88 In addition to neuropeptides, nerve fibers innervating hair follicles produce Shh that signals to a population of cells in the bulge marked by the Hedgehog response gene Gli1.89 The progeny of Shh-responding perineural bulge cells incorporate into healing skin wounds where, notably, they can change their lineage into epidermal stem cells. The perineural niche (including Shh) is dispensable for the follicle’s contribution to wound healing but is necessary to maintain bulge cells capable of becoming epidermal stem cells.89
In turn, hair follicle keratinocytes produce neurotrophic factors that influence perifollicular nerves and stimulate their remodeling in a hair cycle–dependent manner. 88,90 Merkel cells, which are considered neuroendocrine cells, also produce neurotrophic factors, cytokines, or other regulatory molecules. Because Merkel cells are concentrated in the bulge area, some have postulated that these secreted factors may influence the cycling of the hair follicle.91
PERIFOLLICULAR SHEATH
The perifollicular sheath envelops the epithelial components of the hair follicle and consists of an inner basement membrane called the hyaline or vitreous (glassy) membrane and an outer connective tissue sheath. The basement membrane of the follicle is continuous with the interfollicular basement membrane. It is most prominent around the outer root sheath at the bulb in anagen hairs. During catagen, the basement membrane thickens and then disintegrates.
Surrounding the basement membrane is a connective tissue sheath composed primarily of type III collagen. Around the upper follicle, there is a thin connective tissue sheath continuous with the surrounding papillary dermis and arranged longitudinally. Around the lower follicle, the connective tissue sheath is more prominent, with an inner layer of collagen fibers that encircles the follicle surrounded by a layer of longitudinally arranged collagen fibers.
When transplanted under the skin, this perifollicular connective tissue has the remarkable ability to form a new dermal papilla and induce new hair follicle formation. 92 Even when the connective tissue sheath is transplanted to another individual, these follicles survive without evidence of immunologic rejection.
HAIR FOLLICLE CYCLE
Each individual hair follicle perpetually traverses through three stages: (1) growth (anagen), (2) involution (catagen), and (3) rest (telogen). 6 The length of anagen determines the final length of the hair and thus varies according to body site; catagen and telogen duration vary to a lesser extent depending on site. Scalp hair has the longest anagen of 2 years to more than 8 years. Anagen duration in young males at other sites is shorter: legs, 5 to 7 months; arms, 1.5 to 3.0 months; eyelashes, 1 to 6 months; and fingers, 1 to 3 months. In contrast to most mammals, including mice and newborn humans, in adult humans, the hairs of the scalp grow asynchronously. Approximately 90% to 93% of scalp follicles are in anagen, and the rest are primarily in telogen. 65 Applying these figures to the 100,000 to 150,000 hairs on the scalp indicates that approximately 10,000 scalp hairs are in telogen at any given time. However, because an adult loses only 50 to 100 hairs per day, this indicates that telogen is a heterogenous state. The follicles that are shedding their hair shafts are thus in “exogen,” which comprises approximately 1% of the telogen hair follicles (see Fig. 7-2 and later discussion). Hair on the scalp grows at a rate of 0.37 to 0.44 mm/day or approximately 1 cm/month.
HAIR FOLLICLE STEM CELLS
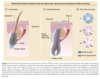
Because the lower portion of the follicle cyclically regenerates, hair follicle stem cells were thought to govern this growth. Historically, hair follicle stem cells were assumed to reside exclusively in the “secondary germ” (see Fig. 7-2), which is located at the base of the telogen hair follicle. It was thought that the secondary germ moved downward to the hair bulb during anagen and provided new cells for production of the hair. At the end of anagen, the secondary germ was thought to move upward with the dermal papilla during catagen to come to rest at the base of the telogen follicle. This scenario of stem cell movement during follicle cycling was brought into question when a population of long-lived presumptive stem cells was identified in an area of the follicle surrounding the telogen club hair. 93 Subsequently, it was shown that the secondary germ is a transient structure that forms at the end of catagen from cells in the lower bulge. 94 The concept that hair follicle stem cells are permanently located in the bulge has now been confirmed using lineage analysis, which showed that the bulge cells give rise to all epithelial layers of the hair follicle. 5,95,96 In line with this, ablation of bulge cells results in destruction of the follicle. 97 These findings support the notion that loss of
hair follicle stem cells in the bulge leads to permanent or cicatricial types of alopecia (see Chap. 88).
Progress has been made in defining subsets of cells within the hair follicle that serve as different stem and progenitor populations. Markers that have been shown through genetic lineage analysis to contribute to the perpetual cycling of the hair follicle include cytokeratin 15 and Lgr5. 97,98 Lgr5, although sometimes touted as an exclusive marker of secondary germ cells, also marks bulge cells. Lgr6, a gene related to Lgr5, is expressed in an area above the bulge in the upper isthmus. The cells marked by Lgr6 migrate to the epidermis during homeostasis and after wounding. 99 In addition to these markers, several others demonstrate the heterogeneity of the hair follicle epithelium.100
Are bulge cells the “ultimate” stem cells within the skin epithelium? For example, do they generate epidermis and sebaceous glands during homeostasis and after wounding? To answer these questions, lineage analysis and transgenic techniques were again used. As illustrated in Fig. 7-3, bulge cells do not normally move to the epidermis, but after full-thickness excision of the skin, bulge cell progeny migrate into the wound during reepithelialization. 5,97 These cells comprise approximately 30% of the cells in the regenerated epidermis. The role of bulge cells in sebaceous gland maintenance is still not clear but is under investigation.
ANAGEN
The formation of a new lower follicle and hair at anagen onset recapitulates folliculogenesis in the fetus. Anagen can be divided into seven stages: (1) stage I—growth of the dermal papilla and onset of mitotic activity in the germlike overlying epithelium; (2) stage II—bulb matrix cells envelop the dermal papilla and begin differentiation, and the evolving bulb begins descent along the fibrous streamer; (3) stage III—bulb matrix cells show differentiation into all follicular components; (4) stage IV—matrix melanocytes reactivate; (5) stage V—hair shaft emerges and dislodges telogen hair; (6) stage VI—new hair shaft emerges from skin surface; and (7) stage VII—stable growth.101
During proliferation and migration of keratinocytes into the dermis to reform the new lower follicle, enzymes such as proteases and collagenases appear at the leading edge of the downgrowth, and growth factors and their receptors are upregulated similar to an epithelial wound. 6 Pathways of keratinocyte differentiation that are seen in the epidermis during wound healing, such as expression of keratin 6, are activated. Mice lacking Stat3, a regulator of cell migration in the cutaneous epithelium, show defects in wound healing and a failure of hair follicles to enter anagen, 102 thus further illustrating the similarity between wound healing and the early events of anagen. Remarkably, the dermal papilla in the midst of this degradative milieu survives and moves downward. Neurocutaneous and vascular networks are remodelled. 90,103 Melanocytes proliferate and repopulate the new hair bulb. 104 Finally, a burst of endothelial proliferation and angiogenesis in the dermal papilla marks the time point when the lower follicle is completely restored and is actively producing the new hair shaft.105
CATAGEN
The onset of catagen is marked by cessation of the mitotic activity of the matrix cells and by wellcoordinated apoptosis in the cyclic portion of the hair follicle. 6,7 Pigment production by melanocytes ceases before matrix cell proliferation stops, thus leading to a nonpigmented proximal end in the telogen club hair (see Fig. 7-2). Melanin is often found in the surrounding dermis and papilla, where it is engulfed by macrophages. The perifollicular sheath collapses, and the vitreous or glassy membrane thickens. The lower follicle retracts upward with the dermal papilla. The perifollicular sheath forms a fibrous streamer composed of fibroblasts, small blood vessels, and collagen. 1 Eventually, the dermal papilla becomes situated immediately below the bulge at the lower portion of the isthmus.
During catagen, the largest follicles, on the scalp, for example, shorten their length from 2- to 5-mm-long structures whose deepest portion, the bulb, extends down into the subcutaneous fat to truncated 0.25- to 0.5-mm follicles in telogen. As the basement membrane around the lower follicle thickens, the dermal
papilla, protected from the surrounding apoptosis and destruction (perhaps because it expresses Bcl-2, an antiapoptotic factor6 ), condenses and begins to move upward to come to rest below the bulge during telogen. The migration of the dermal papilla from the subcutaneous fat to the dermis during catagen is necessary for continued follicle cycling. This is illustrated by the syndrome of atrichia with papules. 106,107 These patients have mutations in either their hairless gene or in their vitamin D receptor gene, in which case they also have rickets. Mice with similar mutations have the hairless phenotype. We know from these mice that folliculogenesis is normal; however, when the follicles enter catagen for the first time, the lower portion of the follicle does not involute and contract properly, and the dermal papilla remains stranded in the subcutaneous fat. 93 Although bulge cells are still present, no new anagen follicles ever form, presumably because the stem cells cannot interact with the dermal papilla.93
The study of mouse mutants has also resulted in several key findings that have increased our understanding of the molecular events at catagen onset. Specifically, Hebert and coworkers 108 discovered that mice lacking the Fgf5 gene have hair that is 50% longer than their wild-type littermates and that mutations in this gene are responsible for the angora phenotype that was described more than 30 years ago. Although these findings were rather unexpected, careful evaluation of Fgf5 expression throughout the normal hair cycle demonstrated that its expression was upregulated in the outer root sheath and hair matrix cells just before the onset of catagen, suggesting that Fgf5 may trigger catagen onset. Interestingly, the follicle still eventually entered catagen, even in the absence of FGF-5, suggesting redundancy in the FGF-5 pathway or an intrinsic finite proliferative capability of the matrix cells. 93 Further studies also demonstrated that other FGF family members and their receptors are expressed during anagen and probably also play a role in the hair follicle cycle. 109 The hair phenotype of FGF-5–deficient mice is substantially reversed by ectopic expression of the antiapoptotic gene bcl-xLx in the outer root sheath, suggesting that regulation of cell survival in the outer root sheath may play a role in control of the hair growth cycle.110
Although it has been known for many years that exogenous EGF administered to sheep results in catagen induction, 111 only through more recent transgenic and knockout studies in mice has the importance of the EGFR system in hair cycle regulation been realized.66,112 For example, knockout mice lacking TGF-α, the major ligand for EGFR, have abnormal hair follicle development and manifest the waved hair phenotype.55,113 When EGFR is functionally downregulated in the basal layer of the epidermis and hair follicle using a dominant negative transgenic strategy, the resulting hair is not only waved but also longer than normal.66 Transition of the hair follicles from anagen to catagen is delayed in these mice. Hair follicles in mouse skin that completely lack EGFR also do not progress from anagen to telogen. 112 Thus, EGFR and its ligand are required for normal hair follicle development and cycling. Given the complexity of the EGFR family,
which includes four receptors (ErbB1–4) and at least six ligands, future studies are needed to clarify the role of individual family members in hair follicle cycling.
In addition to FGF-5 and EGF, neurotrophins and TGF-β1 induce premature catagen. Neurotrophin-3 and brain-derived neurotrophic factor transgenic mice show premature catagen development, and brainderived neurotrophic factor overexpression leads to the shortening of hair length by 15%, most likely via stimulation of proapoptotic signaling through p75 kDa neurotrophin receptor, 114,115 TGF-β1 induces premature catagen in isolated human hair follicles and in mouse skin in vivo, and TGF-β1 knockout mice display delay in catagen onset.116-118
TELOGEN AND EXOGEN
When catagen is complete and a club hair is formed (see Fig. 7-2), the hair follicle prepares the hair for expulsion from the scalp. About 1% of telogen hairs are shed each day. Milner and colleagues 119 have proposed distinguishing hair shedding as a separate phase called exogen. Exogen is a highly controlled and timed event in mammals that shed on a seasonal basis. That exogen is an active stage is supported by Headington’s description of one type of telogen effluvium he termed immediate telogen release. 120 This type of hair loss can be seen soon after starting medications, such as minoxidil, or in response to rapid fluctuations in light–dark cycles. It consists of an increase in shedding of club hairs within weeks of the precipitating event (too soon to be caused by follicles prematurely entering telogen from anagen), suggesting that club hairs that are normally retained in the follicle can be actively shed. The heterogeneity of telogen is further supported by the work of Guarrera and Rebora, 121 who followed individual hairs in situ using macrophotographs for more than 2 years and showed that several months could transpire between hair shedding and regrowth. This “lag period” is normally not present or is very short but often lasts several months in patients with androgenetic alopecia.
HAIR PIGMENTATION

Hair becomes pigmented as a result of a tightly coordinated program of melanin synthesis and transport from the hair bulb melanocytes to differentiating hair shaft keratinocytes. 122-124 This process is strictly coupled to anagen and ceases during catagen and telogen. Numerous signaling molecules, structural proteins, enzymes, cofactors, and transcriptional regulators control hair pigmentation (Figs. 7-4 and 7-5).

HAIR MELANOCYTE DEVELOPMENT
Melanoblasts can be identified in the epidermis of human embryos at 50 days of estimated gestational age before the onset of hair follicle morphogenesis.126-128 These melanocytes originate in the neural crest and migrate first to the dermis and then epidermis. 129 New data reveal that melanocytes in the skin arise from two sources: from neural crest cells migrating in the dorsolateral pathway and from Schwann cell progenitors located in cutaneous nerves. 130 Commitment of neural crest cells to the melanocyte lineage is regulated by Pax3 and microphthalmia transcription factors (Mitf), which stimulate the expression of dopachrome tautomerase (or tyrosinase-related protein 2131 ), an enzyme involved in melanin biosynthesis that also functions as an early melanoblast marker. 128 Subsequent steps of melanoblast development (migration into the dermis and epidermis) are controlled by signaling mechanisms activated through endothelin receptor type B (Ednrb) and c-kit receptor, which are mutated in humans with Hirschsprung disease and piebaldism, respectively, resulting in formation of unpigmented hairs.129
After entering the placode of the developing hair follicle, melanoblasts proliferate and become produce pigment synchronously with the onset of hair fiber formation. 132 Experimental and genetic data suggest that migration of melanoblasts into the hair follicle and their differentiation to pigment producing melanocytes depends on stem cell factor (SCF)/c-kit signaling. SCF is a ligand that binds to its receptor (c-kit). Pharmacologic blockade of c-kit during embryogenesis, as well as genetic ablation of SCF or c-kit in corresponding mouse mutants results in unpigmented hairs.133-135
HAIR FOLLICLE MELANOCYTE STEM CELLS AND PIGMENTPRODUCING MELANOCYTES
Melanocyte stem cells located in the hair follicle bulge generate progeny that repopulate the melanocytes in the new hair bulb formed at the onset of anagen.104,136 Melanocyte stem cells express Trp2, Bcl-2, Pax3, and other melanogenic enzymes (tyrosinase, tyrosinaserelated protein 1131 ) and signaling molecules (c-kit, Ednrb, Sox10, Mitf, and Lef1) 137 are expressed at low levels. Melanocyte stem cells can be first detected in the bulge area during late stages of hair follicle morphogenesis and similar to epithelial stem cells they are quiescent. 136-138 TGF-β signaling plays an important role in controlling melanocyte stem cells entering into a noncycling (dormant) state during hair follicle morphogenesis.
Maintenance of melanocyte stem cells during hair follicle cycling is controlled by TGF-β and Notch signaling pathways. Notch signaling plays a crucial role in the survival of melanocyte stem cells and immature melanoblasts by preventing apoptosis. 140 Cross-talk between the TGF-β pathway and Bcl2 is also important for maintenance of melanocyte stem cells: Bcl2 plays a key role in maintenance of melanocyte stem cells, and Bcl2 knockout mice show progressive hair graying because of the depletion of melanocyte stem cells. 137-139,141,142 However, Bcl2 deficiency may be compensated by overexpression of SCF, which rescues loss of melanocyte stem cells in the hair follicle bulge of Bcl2 knockout mice.
Melanocytes producing pigment are located in the hair bulb above the dermal papilla. 104,124 These cells synthesize and transport melanin to hair shaft keratinocytes and express a full set of enzymes and other proteins involved in melanin biosynthesis including tyrosinase, Trp1, Trp2 (in mice), and pMel17 (in humans). 104,137 Keratinocytes, as pigment recipient cells, produce Foxn1 and its target Fgf2 to identify themselves as the targets for pigment transfer.
HAIR CYCLE DEPENDENT CHANGES IN MELANOCYTES
Hair follicle melanocytes undergo substantial remodeling during hair follicle cycling. 104,124 In telogen, hair follicle melanocytes are found in the bulge, secondary hair germ, and connective tissue. 104 In humans, melanocytes in the telogen hair follicle do not express Trp1- and tyrosinase, do not proliferate, and can be visualized by expression of pMel17. Some of these cells also express c-kit receptor, and others remain c-kit-negative and represent melanocyte stem cells (MCSCs). 104,136,137 Wnt activation in MCSCs drives their differentiation into pigment-producing melanocytes.144 TGF-β signaling is activated in melanocyte stem cells when they reenter the quiescent noncycling state during the hair cycle, and this process requires Bcl2 for cell survival. 139 After wounding or ultraviolet type B irradiation, McSCs in the hair follicle are capable of exiting the stem cell niche and migrate to the epidermis followed by their differentiation into functional epidermal melanocytes in a melanocortin 1 receptor (Mc1r)–dependent manner.145,146
During early anagen, resting melanocytes proliferate, differentiate, and migrate within the hair follicle
synchronously with regeneration of the hair follicle bulb. Hair follicle melanocytes are maximally proliferative during early and midanagen, and their transition to melanogenic competence is accompanied by the appearance of Trp1 and tyrosinase protein.104 However, this process is stringently controlled, and Notch signaling is necessary to prevent differentiation of melanoblasts into pigment-producing melanocytes before they reach the hair bulb, as well as for their proper positioning in the hair matrix.147
Similar to embryonic and early postnatal development, SCF/c-kit signaling plays a critical role in repopulation of the bulb with pigment-producing melanocytes. Whereas c-kit is expressed on proliferating, differentiating and melanocytes producing pigment, overexpression of SCF in the epidermis of transgenic mice significantly increases the number of hair follicle melanocytes and their proliferative activity. 104 Similarly, administration of the ACK45 antibody blocking c-kit signaling dramatically reduces melanocyte number in anagen hair follicles, resulting in hair depigmentation. 104 However, in the next hair cycle, the previously treated animals grow fully pigmented hairs with the normal number and distribution of melanocytes, suggesting that melanocyte stem cells are not dependent on SCF/c-kit.104
During catagen, melanogenic activity in the follicular melanocytes abruptly ceases. Immunohistochemical and electron microscopic data suggest that some pigment-producing melanocytes located above the follicular papilla undergo apoptosis, and others drop into the dermal papilla of the follicle.148,149
MOLECULAR CONTROL OF HAIR COLOR
Follicular melanocytes synthesize pigment via a cascade of enzymatic conversions of phenylalanine or tyrosine into brown-black eumelanin or yellow pheomelanin that requires melanogenic enzymes (tyrosinase, tyrosinase-related proteins 1/2, -glutamyltranspeptidase, peroxidase) and essential co-factors, such as 6-tetrahydrobiopterin. 122,148 The balance between black and yellow pigment synthesis (eumelanin and pheomelanin, respectively) is regulated by signaling through the melanocortin type 1 receptor (MC-1R) that has long been implicated in the controlling hair color.150,151
After binding to MC-1R, α-melanocyte stimulating hormone (α-MSH) stimulates adenylyl cyclase, resulting in elevation of intracellular cyclic adenosine monophosphate (cAMP) levels. This leads to increase of transcriptional activity of MITF that stimulates synthesis of enzymes (tyrosinase, Trp1/2) involved in eumelanin formation. 152,153 Pheomelanin synthesis in the hair follicle melanocytes of mice occurs when MC-1R signaling is inhibited by Agouti signal protein (ASP) that competes with α-MSH in binding to MC-1R.151,152
In mice, ASP expression is positively regulated by BMP signaling, and transgenic mice overexpressing the BMP antagonist Noggin show hair darkening.149 Although ASP is expressed in human skin, its role in human pigmentation remains unclear.153
Recent data also demonstrate the existence of a proopiomelanocortin (POMC)–MC-1R pathway in human hair follicles: MC-1R is expressed by hair follicle melanocytes, and its ligands α-MSH and adrenocorticotrophic hormone (ACTH) are able to promote melanocyte proliferation, dendricity, and pigment production.154 Another POMC-derived peptide, β-endorphin, that interacts with the µ-opiate receptor expressed by hair follicle melanocytes has a similar effect. 154 However, signaling through the µ-opiate receptor may regulate hair pigmentation via modulating the activity of protein kinase C (PKC)-β, a known positive regulator of pigment production.155
Follicular melanocytes are sensitive to aging, which results in their premature loss and hair greying. 156 In contrast to normally pigmented hair follicles, fewer melanocytes are found in the bulb of a grey hair; however, these melanocytes still express tyrosinase and synthesize then transfer melanin to keratinocytes. 157 In addition, a population of inactive melanocytes (melanoblasts including stem cells) in the outer root sheath is markedly reduced in follicles producing grey hairs compared with ones producing pigmented hairs.157 The fact that melanocyte stem cells are damaged in hair follicles producing grey hairs was confirmed in mice by applying ionizing radiation, which triggered premature differentiation of melanocyte stem cells in the follicular bulge into mature, pigment-producing melanocytes followed by their depletion and irreversible hair graying. 158 Deficiency of ATM-kinase, a central transducer of the DNA-damage response, sensitizes MSCs to ectopic differentiation, demonstrating its role in protecting melanocyte stem cells from their premature differentiation.158
influence length, curl, and distribution
fibroblast growth factor-5 (FGF-5), Keratin 71, and R-spondin 2
thicker hair found in Asians is associated with increased activity of what?
Edar


