2.1.2 Biological molecules Flashcards
(127 cards)
what is water comprised off, what is its formula and ratio?
Oxygen and Hydrogen
H2O
2 H : 1 O
how would you descrive the distribution of charge in a water moelcule
an uneven distribution of charge between delta -ve oxygen and delta +ve hydrogen
what is the sign for delta?
𝛿
why is the charge described as uneven?
it is due to the pairs of electrons in each of the oxygen-hydrogen covalent bnds is not equally shared, as the electrons are more stronhly attraced to the oxygen atonms nucleaus than the hydrogen atoms nucleas
Polar definition
Molecules that have an uneven distribution of charge are reffered to as polar
Draw a diagram of a water moeclule, showing the uneven distribution of charge

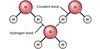
what bond is present IN water molecules
Covalent bonds
what bond is present BETWEEN water molecules
Hydrogen bonds
why are water moleules attracted to each other?
The delta -ve oxygen from one water moelucle will be attracted to a delta +ve hydorgen from another water moelcule
other than water, what other polar groups are there?
Hydroxyl, amine and carbonyl
draw a fiagram of several water moleules, showing the partial charges and the hydrogen bonds
are polar moleucles soulbule or insoaluble and why?
Soluble, becuase water molecules will be attracted to, and will for hydrogen bonds with their polar groups
what is cohesion
when a hydrogen bond forms between to water molecules
what is adhesion
when a water moelcules and a different type of polar molecules for hydrogen bonds
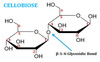
can hydrogen form between two polar moleucles when neither are water?
yes, the same exact thing happens, and example would be two celluose molecules
what is the biological word for polar and water-soluble moleulces?
hydrophilic
hydrophilic deffinition
the physical properties of a molecules which attracts water molecules
are non-polar moelcuels soluble in water and why?
no, they are described as hydrophobic measning they repel water, this is due to the fact they have an even distribution of charge across the moelcules, so they cannot form partial chrages, therfore cannot form hydrogen bonds with water. An example of this is lipids
There are 8 main properties of water you must know what are they?
excellent solvent
effective thermal buffer
high latent heat of vaporisation
low density of ice compared to liquid water
choesion between water molecules
high surface tension
incompressibe
transparent
why is transparancy an important property of water?
it allows light water to pass through water, however its ability to do so depends on the wavelength of light.
this is especially important in aquatic plants and other photoynthetic oganisms which rely on ligh energy to drive photosynthesis (which produces glucose)
why is it good for water to be incompressible?
as water is a liquid, it is incompressible meaning it cannot be forced to decrease in volume.
this is important in insects, which have hydrostatic skeleton to supoort their body.
in plants, cell become turgid when the voume of water in the cell cretes pressure exerted on the cell wall, this is called cell turgor which is vital in enabling the leaves of a plant to be held up to intercept sunlight.
why is it good for water to have high surface tension?
hydrogen bonding from the surface wawter molecules to teh water moleucles below them cretes high surcace tenion, and the water on the top acts a a ‘skin’
this is important becuse small animals such as pnd skaters can move across the surface without breaking through.
why is it good for water to have cohesion between molecules?
water mnolcecules stick togetrher wia hyfrogen bonding. this allows waterto for strong, continuous colums withing a vessel/tube.
These colums have high tensile strength due to the high number of hydrogen bonds between the molecules.
liquids within a vessel will flow from one region to another due to gravity or a hydrostatic pressure difference via mass flow
Effecttive transport mediumn
why is it good that ice is less dense than water
this is due to the fact that water molecules forms a regular semi crystaline structure, with stable intermolecular hydrogen bonds, with the moleucles held far apart from each other.
as ice is less dense than water, ice floats on the surface of water.
Ice on the surace of water insulates the water below, reducing the chances the lower regions freeze. Therefore most aquatic organsism can surviuve even if the upper layers freeze, hence this property creates a stable environment for many species.