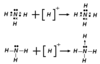Chemical Bonding Flashcards
From sigma bonds and Lewis structure to lattice energy and molecular geometry, use these cards to master the topic of chemical bonding as tested on the MCAT.
What are valence electrons, and how do they affect an atom’s chemistry?
Valence electrons are the electrons in the atom’s highest (outermost) energy subshells. They are chemically relevant because they are the electrons that form chemical bonds.
How would you quickly find the number of valence electrons by using the periodic table?
Valence electron number can quickly be read off of the periodic table, as it equals the column number (counting from the left) that the element is in.
For example, nitrogen is in the 5th column from the left, so it must have 5 valence shell electrons. Nitrogen is 1s22s22p3, and n = 2 is the outermost energy level. Add all of those electrons (s2 and p3) to get valence = 5.
What is the difference in the valence electron configuration of oxygen (O) and silicon (Si)?
Oxygen has 6 electrons in the outermost energy level (2s2 and 2p4).
Silicon has more electrons total, but it only has 4 valence electrons (3s2 and 3p2).
Define:
the octet rule
The octet rule states that atoms desire eight electrons in their valence shells, as this gives them the electron configuration of a noble gas.
Most atoms have to bond with other atoms to acquire this electron configuration.
What energy principle causes two atoms to form a bond between them?
Chemical bonds form because they lower the potential energy of the electron clouds. Electrons are shared between atoms across the bond, allowing the final state to be more stable than the two were alone.
In general, bonding proceeds so that as many atoms as possible gain a full octet of valence shell electrons.
What are the three possible types of interatomic bonds?
-
single bond: one pair of electrons is shared between two atoms
For example, an O-H bond in H2O, or a C-H bond in CH4. -
double bond: two pairs of electrons are shared between atoms
For example, O=O bonds in O2, or C=O bonds in CO2. -
triple bond: three pairs of electrons are shared between atoms
For example, N≡N bonds in N2, or C≡N bonds in HCN.
What are the three notable exceptions to the octet rule?
-
odd-electron species
Molecules like NO must break the octet rule since they have an odd total number of valence electrons to distribute. -
incomplete octets
These are atoms where attaining a full octet would require too many bonds. H, He, Li, Be, B are all considered incomplete octet species due to the high number of electrons they would need. -
expanded octets
Atoms in row 3 or higher, like P or Cl, can hold electrons in their d orbitals. These atoms can hold between 10 and 18 electrons depending on the central atom being bonded.
Why does nitrogen not succeed in getting a full octet in nitric oxide (NO)?
The most electronegative atom will always gain a full octet first.

Nitric oxide has 11 valence electrons. Oxygen is more electronegative, so will end up with a full octet (two sets of lone e-pairs and a double bond with N). Nitrogen will be left with 3 lone electrons (and two bonds), as it is less electronegative, for a total of 7 electrons.
Why will it be unlikely for Li to ever attain a full octet?
Lithium has only one valence electron. It would need to acquire 7 additional electrons in order to have a full octet. This is statistically (and structurally) unlikely.
H, He, Li, Be, B are all elements that will form incomplete octets due to the improbability of them acquiring enough electrons.
Why can phosphorus expand its octet to form 5 bonds to chlorine in PCl5?
Phosphorus can expand its five valence electrons into the 3d block, allowing it to bond to five chlorine atoms and completing those chlorine atoms’ octets.

Elements in the third row of the periodic table and beyond (such as P, Cl, S, Xe, and Ar) often exhibit expanded octets of 10 to 18 electrons. This is due to them expanding into the d-block for greater bonding stability.
What are the three major types of intramolecular bonds?
- ionic bonds: electrons are transferred from a metal to nonmetal
- covalent bonds: electrons are shared between nonmetals
- metallic bonds: electrons “float” between a lattice of metallic nuclei
Note that intramolecular bonds connect atoms of the same molecule, unlike intermolecular forces (like hydrogen bonds).
Define:
an ionic bond
Ionic bonds form when a metal transfers one or more electrons to a nonmetal.

On the MCAT, often this will be a metal from Group I or II transferring electrons to a halogen. The classic example is an “ionic salt” like NaCl. Sodium transfers its one valence electron to chlorine, leaving each with an octet of valence electrons.
How does Coulomb’s law apply to ionic bonds?
Coulomb’s law states that the magnitude of the electrostatic force between two charged particles is directly proportional to the product of the magnitude of each of the charges, and inversely proportional to the square of the distance between the two particles.
This holds for all charged particles, and can be applied to calculate force between the atoms in an ionic bond.
F ∝ q1*q2 / r2
- q1 and q2 = charge magnitude of the ions
- r = distance between the ions
Knowing that Na and Cl have a larger electronegativity difference than K and Br, what does Coulomb’s law predict about the strength of the NaCl bond vs. that of KBr?
NaCl will have stronger bonds between adjacent atoms than KBr, due to the stronger electrostatic force between ions.
How would the force between ions in an ionic compound change if the distance between the adjacent ions was decreased by one-half?
The electrostatic force would be 4 times the original value.
Fbond ∝ q1*q2 / r2
Since new R = r/2:
Fnew ∝ q1*q2 / R2
= q1*q2 / (r/2)2
= q1*q2 / (r2/4)
= Forig/(1/4) = Forig*4
Define:
lattice energy
The lattice energy of an ionic compound is the energy associated with forming a crystalline lattice of the compound from the gaseous ions.
This may also be referred to as “heat of formation.” Note that the value of lattice energy is negative, showing that the formation of an ionic compound is exothermic.
What is the electrostatic energy (Ees) of an ionic bond?
Ees ∝ q1*q2 / r
This holds for all charged particles, and thus can be applied to calculate energy between the atoms in an ionic bond.
- q1 and q2 = charge magnitude of the atom
- r = distance
Note: the value of Ees will always be negative, due to the charges always being opposite in an ionic compound.
How does the energy of an ionic bond change if the distance between the two atoms in the bond doubles?
The final energy will be one-half the initial value.
Eorig ∝ q1*q2 / r
Since new R = 2r:
Enew ∝ q1*q2 / R
q1*q2 / 2r = Eorig/2
What type of bond will exist between atoms in molecules like KBr, CaF2, and LiCl?
These are all ionic compounds, commonly referred to as salts. Ionic bonds will generally form between metals and nonmetals.
Classic examples of ionic compounds will usually include a cation from Group I or II bonded to one or two anions from the halogen family.
Define:
a covalent bond
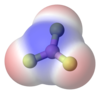
A covalent bond forms when a nonmetal bonds with another nonmetal by sharing electrons between them, resulting in an overlap of their electron orbitals.

The image below of methane shows the polar covalent C-H bonds.
What are the three types of covalent bonds?
- polar covalent bond: electrons are held more closely by the higher electronegative species
- nonpolar covalent bond: electrons are perfectly shared between atoms of the same element
- coordinate covalent bond: entire molecules share electrons for greater net stability
What is the difference in bond strength between molecules in an ionic compound vs. a covalent compound, and how will the compounds’ melting points and boiling points compare?
Ionic bonds create stronger intermolecular forces than pure covalent bonds. Since ionic bonds are extremely polar (more so than any covalent bond, by definition), a strong electrostatic interaction exists between the ions.
The melting point and boiling point will be higher for ionic compounds, as more energy is required to pull the atoms in the bonds apart.
Define:
a nonpolar covalent bond
In a nonpolar covalent bond, both atoms are of the same element. Hence, the electron pair is shared equally. On the MCAT, the only commom pure covalent bonds are the following diatomics: Br2, I2, N2, Cl2, H2, O2, F2 (BrINClHOF).
Note that although some chemistry texts will also include bonds like the C-H bond (since the electronegativity difference is only 0.4 on the Pauling scale), that bond is still slightly polar since electrons favor carbon over hydrogen.
Define:
a polar covalent bond
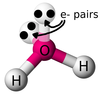
In a polar covalent bond, the electron pair is pulled closer to the more electronegative atom. The result of this is a bond dipole (one end positive, one end negative), hence the term “polar.” The atom that is more electronegative will carry a partial negative charge and the atom that is less electronegative will carry a partial positive charge.
Examples of polar covalent bonds include O-H bonds, N-H bonds, and C-N bonds.
Define:
a coordinate covalent bond
In a coordinate covalent bond between molecules, one atom from one molecule will contribute both electrons to the bond pair. This creates better stability between both molecules.
A common example is a Lewis acid / base pair: NH3 (N donates the electron pair) and H+ (H+ accepts the electron pair). Nitrogen had a full octet to start with, but was very polar until donating the electrons to the bond with hydrogen. H+ didn’t have any electrons, but now will have a full 1s subshell. Both are now more stable.
The first equation below shows all of the actual valence electrons. The second equation is the Lewis diagram representation.