Session 2: Neurones and the Environment of the CNS Flashcards
Describe the network of neurones and glia
Network of neurones with supporting glia
The neurones in the brain form only a small proportion of the overall volume of the brain, and over 50% of the volume of the brain is actually formed by glial cells, acting to support, nourish, insulate, and remove the waste of neurones. There is a ratio of around 10:1 of glial cells to neurones.
Neurones sense changes and communicate with other neurones (around 10^11) neurones
Glia support, nourish and insulate neurones and remove ‘waste’ – (around 10^12) neurones
What are the types of glial cells?
Astrocytes
- Most abundant type of glial cell
- Supporters
Oligodendrocytes
- Insulators
Microglia
- Immune response
What are the roles of astrocytes
Structural support
Quick removal of neurotransmitters
Maintenance of ionic environment
Help to form blood brain barrier but do not actually form it themselves.
Describe the structural support role of Astrocytes
Structural support
Help to provide nutrition for neurones
Neurones require glucose constantly as they cannot store or produce glycogen. To overcome this, the surrounding astrocytes provide a direct source of either glucose or lactose to be transferred to the neurone. Consequently, this action allows for an additional source of energy (from the lactate) for the neurone and during any ischaemia the neurone has a store of lactose of about 5 minutes.
A direct path is seen from the endothelium to neurone by the direct path, yet the astrocyte also has a store of lactate via the glucose-lactate shuttle. This provides the additional source of lactate when required (which means any area of the brain with high energy consumption can receive adequate additional energy via this mechanism).

Describe the quick removal of neurotransmitters role of astrocytes
Remove neurotransmitters (uptake) quickly – control concentration of neurotransmitters (especially important for glutamate (toxic if present in high concentrations)).
Re-uptake: Astrocytes contain transporters specific for neurotransmitters such as glutamate, which can remove the neurotransmitter following an AP, allowing extracellular concentrations of that neurotransmitter to remain low. These neurotransmitters can then be recycled back via the astrocytes by converting them to glutamine.
The maintaining of a low concentration allows for minimal glutamine spread to other receptors of other neurons and preventing any excessively high concentration of glutamate (which can be toxic). Too much glutamate would excite the neurones too much causing Ca2+ entry which can be toxic or it can excite neighbouring neurones (undesired).
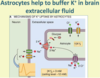
Describe the maintenance of ionic environment role of astrocytes
Maintain ionic environment - extracellular [K+] buffering. A high extracellular K+ concentration around a neuron can result in its depolarisation. Consequently, astrocytes remove K+ ions from the extracellular fluid to keep this ECF concentration low; as a result, astrocytes have a very negative resting membrane potential due to their high intracellular potassium levels. Astrocytes are coupled together by gap junctions.
What is the role of Oligodendrocytes?
Responsible for myelinating axons in CNS
They contain numerous processes that extend out and allow for the myelination of multiple neurones.
(Schwann cells are responsible for myelination in the PNS
Describe the role of microglia
Immunocompetent cells, derived from mesoderm (other glial cells are of ectodermal origin)
Recognise foreign material – become activated
Phagocytosis to remove debris and foreign material
Brain’s main defence system (due to phagocytosis)
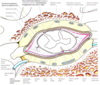
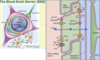
Describe the Blood Brain Barrier
Limits diffusion of substances from the blood to the brain extracellular fluid
Maintains the correct environment for neurones
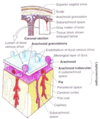
Brain capillaries have
- Tight junctions between endothelial cells. These tight junctions (bound by clodin and occludin proteins) prevent hydrophilic molecules entering through the capillaries.
- Basement membrane surrounding capillaries
- End feet of astrocyte processes. Astrocytes do not form the barrier but they send chemical messages that tell the endothelial capillaries to form tight junctions.
Due to the tight junctions that exist with the capillaries, specialised transporters need to be put in place to allow the movement of needed molecules across the BBB (this appears to be controlled by signals released from the astrocytes). Substances such as glucose, amino acids, and potassium are transported transcellularly across the BBB, allowing their concentrations to be controlled. Gaseous molecules and H2O can diffuse freely across the BBB, as will any lipophilic molecules
How is the CNS Immune Privileged?
: Immune privileged (immune specialised)
Does not undergo rapid rejection of allografts
Rigid skull will not tolerate volume expansion – too much inflammatory response (i.e. swelling) would be harmful
Microglia can act as antigen presenting cells (to T cells) as well as phagocytose foreign material
T-cells can enter the CNS
- CNS inhibits the initiation of the pro-inflammatory T-cell response - The CNS has a regulated inflammatory response, whereby T-cells are able to enter the CNS yet their inflammatory T cell response is significantly limited. Any inflammatory expansion in the CNS would not be tolerated due to the rigidity of the skull, so inflammatory responses are limited.
Immune privilege is not immune isolation, rather specialisation (to control immune response)
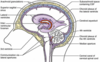
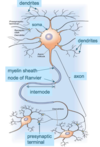
Describe the typical structure of a neurone
Typical neuronal structure
Cell soma
Dendrites (normally receive synaptic input)
Axon
Terminals
The axonal hillock is where the action potential is generated to pass along the axon.
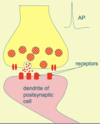
Describe neurotransmitter release
Neurotransmitter release: the synapse
Action potential leads to depolarisation in the presynaptic terminal which opens voltage-gated Ca2+ channels. Influx of Ca2+ ions enter the terminal.
Vesicles fuse and release transmitter.
Neurotransmitter diffuses across the synaptic cleft and binds to receptors on the postsynaptic membrane
What does the postsynaptic response depend on?
Postsynaptic response: depends on
Nature of transmitter
Nature of receptor
Ligand-gated ion channels
G-protein-coupled receptors
A single neurotransmitter can have a number of different receptors e.g. ACh has both nicotinic and G-Protein Coupled Receptors.
What are the types of neurotransmitters in the CNS?
>30 neurotransmitters have been identified in the CNS
Can be divided into 3 chemical classes:
Amino acids: glutamate, GABA, glycine
Excitatory amino acids include glutamate (main one). It is the major excitatory neurotransmitter – over 70% of all CNS synapses are glutamatergic (most abundant) and are present throughout the CNS.
Inhibitory amino acids include GABA and glycine
Gaba is the main inhibitory transmitter in the brain
Glycine acts as an inhibitory neurotransmitter mostly in the brainstem and spinal cord
Biogenic amines: acetylcholine, noradrenaline, dopamine, serotonin (5-HT), histamine
Mostly act as neuromodulators, confined to specific pathways (not present throughout the CNS)
Peptides: dynorphin, enkephalins, substance P, somatostatin, cholecystokinin, neuropeptide Y. Dynorphin, enkephalins and substance P are all involved in pain regulation. Somatostatin, cholecystokinin and neuropeptide Y also have roles in the GI system.
Describe Glutamate Receptors
Glutamate receptors can be either Ionotropic or metabotropic
- Ionotropic have an integral ion channel – permeable to Na+ and K+ (and in some cases Ca2+ - crucial for NMDA function) ions
AMPA receptors: Na+/K+ (responsible for very fast response)
Kainate receptors: Na+/K+
NMDA receptors: Na+/K+ and Ca2+ (slower response)
Activation of ionotropic channels causes depolarisaton – increased excitability
- Metabotropic: mGluR1-7
G-protein coupled receptor
Linked to either:
Changes in IP3 and Ca2+ mobilisation
Or inhibition of adenylate cyclase and decreased cAMP levels
Describe Fast Excitatory Responses
Excitatory neurotransmitters cause depolarisation of the postsynaptic cell by acting on ligand-gated ion channels
Excitatory postsynaptic potential (EPSP)
Depolarisation causes more action potentials to be triggered.

Describe Glutamergic Synapses
Glutamatergic synapses have both AMPA and NMDA receptors (tend to exist together at the synapse)
AMPA receptors mediate the initial fast depolarisation
NMDA receptors are permeable to Ca2+
NMDA receptors need glutamate to bind and the cell to be depolarised to allow to flow through the channel – slower response but this avoids Mg2+ ions plugging the hole.
Also glycine acts as a co-agonist
Entry of Ca2+ during NMDA receptor activation can cause cell damage if intracellular Ca2+ levels become too high. Consequently, excessive amounts of glutamate can cause cell death, known as excitotoxicity.
Describe the role of Glutamate receptors in learning and memory
Glutamate receptors, synaptic plasticitiy and excitotoxicity
Glutamate receptors have an important role in learning and memory
Activation of NMDA receptors (and mGluRs) can up-regulate AMPA receptors
Strong, high-frequency stimulation causes long term potentiation (LTP) – strengthening a synapse => creates a memory (shared synaptic response)
Ca2+ entry through NMDA receptors important for induction of LTP
Too much Ca2+ entry through NMDA receptors causes excitotoxicity
- Too much glutamate – excitotoxicity
- The spread of depolarisation could activate NMDA receptors around the infarct in stroke, causing further damage.