Gupta - Pathology Flashcards
What do you see here?

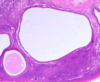
- First-trimester chorionic villi
- Composed of delicate mesh of central stroma surrounded by two discrete layers of epithelium:
1. Outer layer of syncytiotrophoblast (group of cells; two arrows) and
2. Inner layer of cytotrophoblast (single cells; arrow) - Smaller, more vascular, less trophoblast thickness as the pregnancy progresses
What is this?

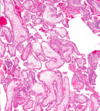
- Third-trimester chorionic villi
- Composed of stroma with:
1. Dense network of dilated capillaries
2. Surrounded by markedly thinned-out syncytiotrophoblast and cytotrophoblast - REMEMBER:
1. Syncytio: think groups of cells
2. Cyto: think single cells
What kind of growth restriction do fetal abnormalities cause? Specific causes?
- SYMMETRIC: all organ systems are similarly affected
- Caused by chromosomal disorders, congenital anomalies, and congenital infections:
1. TORCH group of infections; usually transplacental -
a. Toxoplasmosis: usually 1st infection is the one that causes most problems (i.e., if mom has never been exposed before)
b. Rubella
c. Cytomegalovirus (CMV)
d. Herpesvirus (HSV)
2. Other viruses/bacteria, i.e., syphilis bacteria, listeria - NOTE: wear gloves if working in the garden while pregnant

What kind of growth restriction do placental abnormalities cause? Specific causes?
- ASYMMETRIC: spares brain (via a down-regulation of growth in latter 1/2 of gestation)
- Uteroplacental insufficiency may result from:
1. Umbilical-placental vascular anomalies (like single umbilical artery, abnormal cord insertion, placental hemangioma),
2. Placental abruption (detachment from uterus),
3. Placenta previa (covers opening of mom’s cervix),
4. Placental thrombosis and infarction,
5. Placental infection, or
6. Multiple gestations - NOTE: 3rd trimester loss is usually placental insufficiency
What kind of maternal abnormalities may cause fetal growth restriction?
- Those that result in DEC placental blood flow
1. Vascular diseases, like preeclampsia (toxemia of pregnancy) and
2. Chronic hypertension, are often the underlying cause - Usually some kind of vascular issue
What is spontaneous abortion? Causes?
- MISCARRIAGE: pregnancy loss <20 wks gestation
1. Fetal chrom anomalies: approx 50% of early abortuses
2. Maternal endocrine factors: luteal-phase defect, poorly controlled diabetes, other uncorrected endocrine disorders
3. Physical defects of uterus: submucosal leiomyomas, uterine polyps or malformations, may prevent or disrupt implantation
4. Systemic disorders affecting maternal vasculature: antiphospholipid Ab syndrome, coagulopathies, and HTN - NOTE: families get very upset about losing the fetus early, but this may be a “good” thing in many cases, i.e., due to a chromosome abnormality
When might ascending infections cause pregnancy loss?
- 2nd trimester
- Losses due to fetal and maternal anatomic abnormalities are also common in the 2nd trimester
What is this?

- Listeria: necrotizing intervillositis
-
Non-pasteurized cheeses and milk (KNOW THIS: may get a question about it) -> if the infection is bad enough, you may lose this pregnancy
1. Outbreaks in some places where people eat a lot of unpasteurized cheeses (also cold cuts)
What is going on here?

- Chorioamnionitis: maternal inflammatory response to intervillositis
- These are ascending infections, so often maternal inflammatory response (not placenta)
- Lots of inflammation: would also call this deciduitis (this is acute inflammation -> mostly polys)
- NOTE: layers here are amnion, chorion, decidua
What do you see here?

- Necrotizing funisitis: due to Candida, in this case
- Punctate, 1-2mm yellow-white nodules on the cord (Candida always looks the same)
- Generally track the coils of the cord vessels, and are noted initially along the perimeter of the umbilical vein (3 vessels: 2 aa, 1 vein)
- Established, or long-standing infections can cause tissue necrosis and accumulation of cellular debris (necrotizing funisitis)
- False knot in the cord here
What is this?

- Subamniotic microabscesses typical of Candida funisitis
- Very characteristic, but not specific for this organism
- Inflammation of the umbilical vessels (vasculitis) and cord substance (funisitis) occurs in response to the infection -> FETAL INFLAMMATORY RESPONSE
-
Wharton jelly: supporting substance in umbilical cord that looks like jelly grossly, but pink ground substance in this micro
1. Right side of the image is the outside of the umbilical cord
2. Artery on the left
What is this?

- Candida pseudohyphae with GMS silver stain in subamniotic foci
- Funisitis: umbilical cord with subamniotic microabscesses
- Inflammation located at the periphery or surface of the umbilical cord may be the primary pattern seen (peripheral funisitis)
- Fungal ball
What is phlebitis?
- Inflammation of the umbilical vein
- Can be seen separately from arteritis (inflammation of umbilical arteries)
What do you see here?

- Chronic villitis with CMV: likes vascular endothelium
- Nuclear AND cytoplasmic inclusions (herpes would only have nuclear inclusions)
- This case is SEVERE
What is this?

Chronic villitis with CMV
What is going on here?

- Parvovirus B19: erythema infectiosum (slap cheek)
- Viral inclusions in early erythroid precursors
- Bone marrow sample b/c don’t see nucleated red cells in peripheral system, unless something is wrong
What do you see here? Arrow?

- Parvovirus B19: erythema infectiosum
- Erythroblasts in lumen of capillary vessels of the placental villi show eosinophilic nuclear inclusions
An image of fetal membranes are shown. Iron stain was negative. The histologic finding indicates what?

A. Meconium was present in the amnionic cavity
B. Placental abruption
C. Congestive heart failure in the fetus
D. Acute chorioamnionitis
A. Meconium was present in the amniotic cavity
- Passage of meconium in utero due to bowel peristalsis and relaxation of anal sphincter -> may be indicative of fetal distress
- Meconium components diffuse into placenta and cord, leading to vasoconstriction + hypoperfusion; damage to fetus INC with length of exposure
- Neonates are at risk for meconium aspiration
- Meconium is unlikely in fetuses <30 wks gestation
-
Meconiophages: brown-green pigment, usually right below amnion, in the chorion
1. Top a little bit of amnion layer (low, cuboidal epithelium), then chorion, then decidua (big, fluffy, pink cells)
2. Hemosiderin-laden macros would have been positive for iron
What is twin-twin transfusion syndrome?
- A complication of monochorionic twin pregnancy
- Monochorionic twin placentas have vascular anastomoses that connect twin circulations, and in some cases these connections include one or more INC blood flow to one twin at expense of the 2nd, one twin will be underperfused, while the second will be fluid overloaded
- If severe, this may result in the death of one or both fetuses
- Most twins Di/Di, so this syndrome is RARE

What may have happened here?

- Twin-twin transfusion syndrome: a complication of monochorionic twin pregnancy
- Monochorionic twin placentas have vascular anastomoses that connect twin circulations, and in some cases these connections include one or more INC blood flow to one twin at expense of the 2nd, one twin will be underperfused, while the second will be fluid overloaded
- If severe, this may result in the death of one or both fetuses

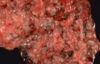
What is the most common site of ectopic pregnancy? Causes?

- Approximately 90% of cases in extrauterine fallopian tube
- Can rupture (gross image on front of this card), leading to hemoperitoneum, which is dangerous
- 50% are idiopathic, and the other 50% may be infection, leiomyoma causing obstruction, etc.
- Takes a “villous” to prove ectopic pregnancy; chorionic villi float on water

Describe each of these abnormalities of placental implantation (A-D).

- Fertilized ovum normally implants in uterine fundus
A. Normal placenta
B. Placenta previa: very low lying placenta, or one that covers the os -> severe hemorrhage can result, w/cervical dilation and passage of the baby through the birth canal
C. Placenta accreta: no formation of a normal decidual plate -> chorionic villi extend into myometrium, and placenta can’t separate normally post-delivery; severe hemorrhage
D. Abruptio placenta: premature separation of placenta prior to delivery, with formation of a retroplacental blood clot between decidua and uterine wall -> blood supply of O2 and nutrients to fetus compromised to greater degree with INC size of the abruption
What do you see here?

- Placenta accreta: NO formation of normal decidual plate -> chorionic villi extend into myometrium, and placenta can’t separate normally post-delivery, leading to severe hemorrhage
- Microscopically, the placental villi interdigitate directly with the uterine myometrium, without an intervening decidual plate
How is placental invasion into the myometrium classified (chart)?

The following gross and microscopic images show what pathology?
A. Placenta previa
B. Normal placenta
C. Abruptio placenta
D. Placenta accrete
E. Vasa previa

C. Abruptio placenta
- Retroplacental blood clot in gross image
What is this?

-
Amnion nodosum: seen in placentas affected by oligohydramnios, which may be associated with fetal renal agenesis and pulmonary hypoplasia
1. May be due to desquamated skin or membrane injury - GROSS: multiple yellow-tan superficial amniotic lesions (0.2 to 0.4 cm), usually near insertion of the umbilical cord
- Not really a bad thing, unless it is associated with oligohydramnios -> just a collection of squaffed off squamous cells, and maybe some fibroblasts
What is going on here?

-
Amnion nodosum: seen in placentas affected by oligohydramnios, which may be associated with fetal renal agenesis and pulmonary hypoplasia
1. May be due to desquamated skin or membrane injury - MICRO: nodules of protuberant eosinophilic fibrinous material with entrapped squamous cells
1. Associated w/stratified squamous metaplasia - Not really a bad thing, unless it is associated with oligohydramnios -> just a collection of squaffed off squamous cells, and maybe some fibroblasts
What happened to this little guy?

- Potter’s sequence: clubbed feet, pulmonary hypoplasia, and cranial anomalies
- Oligohydramnios experienced in utero
What may have caused the difference in size of these placentas?

- Preeclampsia: HTN and large amount of protein in the urine -> usually in 3rd trimester of pregnancy and gets worse over time (feared outcome is eclampsia = seizures)
- Most placentas are smaller than expected
- Infarcts and retroplacental hematomas are also more common
What do you see here? When are these more likely?

- Placental infarct: small infarct may be of no consequence to the fetus, but if >1/3 or 1/2 of the placental parenchyma is infarcted or lost in some fashion, blood supply to fetus can become severely compromised and fetal demise may occur (via utero-placental insufficiency w/multiple infarcts)
- Infarcts AND retroplacental hematomas are more common in PREECLAMPSIA
- Bread loafing for placenta section: maternal vessels on top, membrane on bottom, and pale area is an infarct
What is the difference between these two images?

- Preeclampsia
- LEFT: villi small, and syncytiotrophoblasts have thinned out (as they should)
- RIGHT: villous ischemia (increased syncytial knots) in the placenta
1. A little hemorrhage; don’t look as healthy
2. Only some blood vessels, and “nubbins” of syncytiotrophoblasts
What might be going on in this maternal vessel?

- Fibrinoid necrosis of the vessel wall: strong eosinophilic staining areas
- PREECLAMPSIA
- These are maternal vessels in the decidua
-
No cell nuclei, and decidua surrounding it (big, pink, fluffy cells)
1. Pink and amorphous (necrotic)
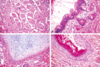
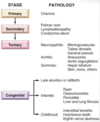
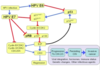
Why are hyatidiform moles important to recognize?
- Because they are associated with an increased risk of persistent trophoblastic disease (invasive mole) or choriocarcinoma
- Invasive: trophoblasts go into uterine wall
- Attached flow chart is important for pathology (not important for us to know yet) -> partial moles much more difficult than complete ones because often diagnosed very early

What is the origin of complete/partial hydatidiform moles? What hormone level could help you distinguish between the 2?
-
Complete: egg that has lost female chromosomes, so genetic material is completely paternally derived
1. NO fetal parts - Partial mole: fertilization of an egg with two sperm
-
hCG: compared to normal levels for gestation
1. Complete mole: very elevated
2. Partial mole: slightly elevated

What is this?

Gross image of a twin placenta
What is preeclampsia?
-
Vasculature problem: may be b/c trophoblasts don’t sufficiently invade/stimulate the spiral arteries in the uterus -> hypoxic state for the fetus, which is reflected in the gross and micro changes
1. Big, dilated, torturous blood vessels on fetal surface on left (attached image)
2. Right placenta much smaller: DEC blood supply, nutrients to fetus + more likely to get more infarcts and retroplacental hematomas

What do you see here?

-
Complete mole: no embryo, and no normal placenta
1. Marked villous enlargement, edema, and circumferential trophoblast proliferation - Villi get very large, and edematous, aka hydropic
- hCG very elevated (above what would be normal for that stage of the pregnancy) in these women because way too many syncytiotrophoblasts
What is this?

- Snowstorm appearance of complete mole on US
- Buzzword
- REMEMBER: no embryo, and no normal placenta
1. Elevated hCG for the corresponding stage of pregnancy
What do you see here?

-
Normal first trimester placenta: chorionic villi are large and covered by two layers of cells:
1. Cytotrophoblast and syncytiotrophoblast (syncytio make hCG) - Blood vessels in the villi are not prominent
What is this?

-
Partial mole: some villi appear normal; others are swollen, avascular, and grape-like (though not as large as a complete mole)
1. Minimal trophoblastic proliferation - hCG won’t be as high as with the complete mole
- Can be really hard to differentiate these from normal villi microscopically (b/c detected so early)
- Will karyotype if clinical suspicion is very high (looking for TRIPLOIDY)
What is this?

Complete mole: NO normal villi here
What is this?
- Partial mole: normal villi are pink, fluffy, light tan (vs. grape-like clusters of the molar villi)
What might this be?

-
Gestational choriocarcinoma: malignant neoplasm of trophoblastic cells from a previously normal or abnormal pregnancy, like an extrauterine ectopic pregnancy or complete mole
1. Complete moles more likely to progress to this than partial, but still only about 2-3% risk - Rapidly invasive and metastasizes widely, but once identified responds well to chemotherapy
1. Very hemorrhagic
2. >50% have metastasized by the time they are identified - NOTE: persistent trophoblastic disease does not metastasize, but pieces of it may embolize to lung or other parts of body
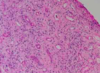
What is this?

- Choriocarcinoma: soft, fleshy, yellow-white tumor with large, pale areas of necrosis and extensive hemorrhage
- Histo: proliferating syncytio and cytotrophoblasts
1. Mitoses abundant and sometimes abnormal - Invades underlying myometrium, freq penetrates blood vessels, and in some cases extends out onto the uterine serosa and into adjacent structures
- Chorionic gonadotropins (hCG) very high!!!!
What are the histo and clinical features of complete hydatidiform mole?
- HISTO: large, avascular villi with trophoblastic proliferation
- CLINICAL: occurs when fertilized ovum contains only paternal chromosomes (usually 46, XX karyotype)
1. Marked uterine enlargement
2. “Snowstorm” effect w/no fetus on US
3. Some give rise to choriocarcinoma (2-3%)
What are the histo and clinical features of partial hydatidiform mole?
- HISTO: some villi enlarged, w/minimal trophoblast proliferation
- CLINICAL: typically triploid (69, XXX or 69, XXY or 69, XYY)
1. Malformed fetus present, but rarely goes to term
2. Rarely gives rise to choriocarcinoma
What are the histo and clinical features of choriocarcinoma?
- HISTO: malignant proliferation of syncytio-trophoblast with no villi
1. Often hemorrhagic
2. NO fetus is present - CLINICAL: hCG levels are often extremely high
1. Can metastasize
2. Many are sensitive to chemotherapy
What are the histo and clinical features of a placental site trophoblastic tumor?
- HISTO: rare, localized proliferation of intermediate trophoblast that can produce a grossly visible nodule
- CLINICAL: most are benign, with rare malignant cases
What are some of the hazards of prematurity?
- May give rise to:
1. Neonatal respiratory distress syndrome, also known as hyaline membrane disease
2. Necrotizing enterocolitis (attached image)
3. Sepsis
4. Intraventricular and germinal matrix hemorrhage

What is going on here?

- Necrotizing enterocolitis (NEC): potential hazard of prematurity
- LEFT: entire small bowel markedly distended, with a perilously thin wall (impending perforation)
- RIGHT: congested portion of ileum corresponds to areas of hemorrhagic infarction and transmural necrosis microscopically
1. Submucosal gas bubbles (pneumatosis intestinalis) can be seen in several areas (arrows on bottom left)
What is the difference between these two tissues?

-
Hyaline membrane disease (neonatal respiratory distress syndrome) in the image on the left
1. Alternating atelectasis and dilation of alveoli
2. Eosinophilic, thick hyaline membranes lining the dilated alveoli - Normal fetal lung on the right

What is fetal hydrops? What are some of the causes?
- Accumulation of edema fluid in the fetus during intrauterine growth
- CAUSES: CV, chromosomal, fetal anemia (immune, Parvo B19, homo alpha-thalassemia), twin-twin, etc.
1. Immune hydrops: major antigens known to induce clinically significant immuno rxns are certain Rh Ags and ABO blood groups
a. 2nd and subsequent pregnancies in Rh-(-) mom w/Rh-(+) dad
b. O- moms

What happened here?

- Fetal hydrops: generalized accumulation of fluid in the fetus
- RIGHT image: fluid accumulation is particularly prominent in the soft tissues of the neck, and this condition has been termed cystic hygroma
1. Characteristically seen, but not limited to, constitutional chromosomal anomalies such as 45,X karyotypes (Turner’s syndrome)
What is SIDS? Risk factors?
-
Sudden death of infant <1 year of age that remains unexplained after thorough case investigation, incl performance of a complete autopsy, examination of the death scene, and review of the clinical history
1. Aka, sudden unexpected infant death (SUID) - “Triple-risk” model that postulates the intersection of three overlapping factors:
1. Vulnerable infant,
2. Critical devo period in homeostatic control,
3. Exogenous stressor - NOTE: babies should sleep on their backs
Klinefelter syndrome
- XXY
- Eunucoid (indeterminate sex) features
- Loss of sertoli cells = loss of inhibin and INC FSH
1. FSH stimulates aromatase in gonads, INC estrogen
2. Female secondary sex characteristics at puberty
Androgen insensitivity syndrome
- XY phenotypic “female;” presents w/amenorrhea
- Normal testis: leydig cells make testosterone
1. Loss of testosterone receptor activity
2. No testosterone effect = NO development of male reproductive structures - No conversion of testosterone to DHT = no external developed male genitals
- XY means presence of MIF (sertoli cells), inhibits female reproductive organs, however, distal 2/3 forms due to estrogen (also normal breast)
Premature ovarian failure
- Female <40 with amenorrhea
- Loss of entire ovarian function
- DEC estrogen and inhibin feedback results in increased FSH and LH
- Progesterone withdrawal test is negative for bleeding
PCOS
- Female presents with amenorrhea and hirsutism
- INC LH with intact ovary -> INC testosterone (hirsutism)
- INC T converted to estrogen by aromatase, so INC LH (like LH surge) and INH FSH, leading to NO follicle development
1. Follicle becomes a cyst - Progesterone test is positive for bleeding
Turner syndrome
- 46, XO: monosomy
- Short, primary amenorrhea, lymphedema at birth
- Shield chest, wide nipples
- Webbed neck and low hair line
- Coarctation of the aorta
- Streak ovaries –> little estrogen
1. Elevated FSH and LH
Kallman syndrome
- Defect in hypothalamic production of GnRH
- Associated with anosmia: inability to perceive odor
- Delayed puberty
What is the progesterone withdrawal test?
- Give patient w/amenorrhea medroxyprogesterone acetate (Provera) 10 mg daily for 5-10 days or one IM injection of 100-200 mg of progesterone in oil
- If patient experiences bleeding after the progestin, she has estrogen present but is not ovulating (anovulation)
- If no withdrawal bleeding occurs, either patient has very low estrogen levels or there is a problem with the outflow tract such as uterine synechiae (adhesions) or cervical stenosis (scarring)
Appreciate this.

Good job!
What are the most common palpable breast lesions?
- Cysts
- Fibroadenomas
- Invasive carcinomas

Where are most carcinomas located in the breast? Do they metastasize?
- About 50% are located in the upper outer quadrant
- Some do metastasize, and the majority of those that do will have done so by the time they reach a size that can be palpated -> around 2-3cm
1. This is why mammography has become such a big deal -> helps us catch lesions earlier - Note: ductal and lobular carcinomas come from the same cell type, but appear different
What % of breast masses in women are malignant (2 age groups)?
- 10% in women younger than age 40 are malignant
- 60% are malignant in women over 50
- Breast cancer risk goes starts going back down after age 65 (see attached)

What are the 4 key symptoms of breast disease (image)?

What are the 4 main presentations of breast cancers?
- ABNORMAL MAMMOGRAM

What are fibrocystic changes? Categories and specific types (image)?
- Benign cyst formation and fibrosis
- Most common abnormality in premenopausal women
- Cyclic changes with menstrual cycle, especially fibroadenomas
1. Oral contraceptive pills (OCP) seem to DEC risk - Subdivided into proliferative (slightly more risk than non-proliferative to progress to something malignant, but still minimal) and non-proliferative
- IMAGE: UDH = usual ductal hyperplasia, ADH = atypical ductal H, ALH = atypical lobular H

What are non-proliferative fibrocystic changes?
- Cysts and fibrosis
- May have calcifications picked up on mammography
- Cysts may undergo apocrine metaplasia
What do you see here? How do you know?

- Gross image of fibrocystic changes
- Normal breast has a lot of yellow, fatty tissue, but fibrosis is white and springy (going to bounce back if you squish it in)
1. Cancer would be more gritty, and not have the same kind of bounce - Large cyst at the top left
- Blue dome cysts: no histologic correlation -> just a gross observation (middle-right of the image)
What is this?

- Benign fibrocystic changes: large, cystic structures
- Cystic and apocrine metaplasia near the bottom
- Some calcification
- Can do stains for myoepithelial cells because these are lost in breast cancer
What do you see here?

- Apocrine metaplasia: non-proliferative fibrocystic change
- Bright, bubblegum pink color to the cells
- Fibrosis instead of the typical breast fat and stroma
What do you see here?

- Usual ductal hyperplasia (UDH): proliferative fibro-cystic change
- Lumen filled by a heterogeneous, mixed population of luminal and myoepithelial cell types
1. Two cell layers: NOT DCIS (an invasive cancer that would necessitate lumpectomy or chemo) b/c myoepithelial layer present
2. Cells swarming, hanging out together: all on top of e/o, rather than individualied (as in atypia) - Irregular slit-like fenestrations prominent at periphery

What is this?

- Atypical ductal hyperplasia: proliferative fibrocystic change -> atypia b/c cells starting to dissociate from e/o rather than be all on top of e/o
- Histo resemblance to ductal carcinoma in situ (DCIS), but not classified as such due to its SIZE
1. >2mm or 2 ducts = DCIS (really ugly cytology or necrosis may also push patho to dx this as DCIS)
2. Still have myoepithelial cells - Relatively monomorphic proliferation of regularly spaced cells, sometimes with cribriform spaces
1. No slit-like spaces, but rather a cookie-cutter appearance (and Roman bridges; archways)
What do you see here?

- Atypical ductal hyperplasia: proliferative fibrocystic change -> atypia b/c cells starting to dissociate from e/o rather than be all on top of e/o
- Histo resemblance to ductal carcinoma in situ (DCIS), but not classified as such due to its SIZE
1. >2mm or 2 ducts = DCIS (really ugly cytology or necrosis may also push patho to dx this as DCIS)
2. Still have myoepithelial cells - Relatively monomorphic proliferation of regularly spaced cells, sometimes with cribriform spaces
1. No slit-like spaces, but rather a cookie-cutter appearance (and Roman bridges; archways)
What is this?

- Atypical lobular hyperplasia: cells identical to those of lobular carcinoma in situ, but the cells do not fill or distend more than 50% of the acini within a lobule
- Can become ductal or lobular carcinoma
- Don’t do anything with this right now per standard of care
What do you see here?

- Sclerosing adenosis: proliferative changes
- Involved terminal duct lobular unit enlarged, and acini compressed and distorted by dense stroma
1. Dilated glands - Calcifications present within some of the lumens
- Unlike carcinomas, acini are arranged in a swirling pattern, and the outer border is well circumscribed
- Myoepithelial marker (+) = “good to go” in calling this sclerosing adenosis rather than carcinoma
What is this?

- Radiograph: irregular central mass with long radiodense projections (needle-localized lumpectomy in this image)
- Grossly: solid and has irregular borders, but not as firm as an invasive carcinoma; tan/yellow instead of grey/gritty of cancer
-
Micro: central nidus of small tubules in a densely fibrotic stroma w/numerous projections containing epithelium with varying degrees of cyst formation and hyperplasia
1. Thick, keloid-like collagen with ducts radiating from central location
How do fibrocystic changes affect cancer risk?
-
Minimal to no INC risk:
1. Non-proliferative changes: cysts, fibrosis, apocrine metaplasia
2. Mild hyperplasia (UDH) -
Slightly INC (1.5-2-fold):
1. Moderate to florid hyperplasia (w/o atypia)
2. Ductal papilloma
3. Sclerosing adenosis - Significant risk (5-fold__): ADH, ALH, DCIS, LCIS
What inflammatory processes can affect the breast (5)?
- Acute mastitis: breastfeeding; staph aureus
- Fat necrosis: trauma, might calcify and appear abnormal on a mammography (attached image: shriveled and ghost-like, with lipid-laden macros coming in to clean up everything)
- Mammary duct ectasia: nonbacterial chronic inflammation
-
Lymphocytic mastitis: diabetes, possible autoimmune (not common; incidental finding)
1. Comes in and encircles the ducts - Granulomatous mastitis: TB, injections, breast implants, sarcoidosis

What are the 4 tumors of the breast?
- Fibroadenoma
- Phyllodes tumor
- Intraductal papilloma
- Carcinoma
What is a fibroadenoma?
- MOST COMMON benign neoplasm of F breast
- Usually relatively small, but can get pretty large
1. Juvenile FA: can rapidly enlarge in teens - Biphasic: glands AND stroma, but only stroma truly neoplastic
- 1-10cm fibroepithelial lesion
What is this?

- Fibroadenoma: usually very well circumscribed
- Not fixed (mobile); cancer tends to be more fixed because it can be invasive
- BIPHASIC: ducts and stroma (stroma squeezes the ducts closed/down)
What do you see here?

- Fibroadenoma: usually very well circumscribed
- Not fixed (mobile); cancer tends to be more fixed because it can be invasive
- BIPHASIC: ducts and stroma (stroma squeezes the ducts closed/down)
What is this?

- Phyllodes tumor (stromal): distinguished from FA’s on basis of higher cellularity & mitotic rate, nuclear pleomorphism, stromal overgrowth, and infiltrative borders -> leaf-life pattern
What is this?

- Phyllodes tumor: stromal
- Most of these are NOT malignant, but the ones that are will look kind of like a fibrosarcoma with lots of stromal overgrowth and mitotic figures
- Cigar-shaped cells
What do you see here?

- Malignant phyllodes tumor: most of these are NOT malignant, but this one is
- >10 mitotic figures per 10 high per fields AND stromal > ductal overgrowth
- Densely packed anaplastic stromal cells and infiltrative border
- Tumor necrosis, heterologous stromal elements, may have cellular stroma only around blood vessels
What is an intraductal papilloma? How does it present?
- Papillary projection inside the duct
- Can present with nipple discharge, and can rarely induce nipple retraction
- Double layer epithelium helps differentiate between papillary carcinoma, but not always so black and white
- Have to be really careful with diagnosing these because biopsy may alter the integrity of the sample
What is this?

- Intraductal papilloma: central fibrovascular core extends from the wall of a duct.
- The papillae arborize within the lumen and are lined by myoepithelial AND luminal cells (two cell layers distinguishes this from carcinoma)
- Fibrovascular stalk
- Location is one of the keys: sub-areolar
What is the lifetime risk of breast cancer for women?
- 12.3% (1 in 8 women)
- Most common cancer in women, and 3rd most common cause of death from cancer
What does this graph mean?

We have not made much progress in regards to limiting deaths from breast cancer
How does race affect breast cancer mortality?
- African-americans die at a much higher rate (then Caucasians)
What are the risk factors for breast cancer?
- Age: INC through life
- Geographic variations: environmental, higher in US and Northern Europe
- Race: AA
- Exogenous estrogens: shift to combined estrogen + progesterone created drop in new cases
-
Radiation
1. Genetics: ataxia telangiectasia -> likelihood of breast cancer and radiation - Others include obesity (estrogen), alcohol, and high fat diet
What are the most important factors in predicting breast cancer outcomes?
- Tumor size
- Lymph node status
What is the pathogenesis of breast cancer?
- Incompletely understood, but multifactorial:
1. Genetics
2. Hormonal influences
3. Environmental influences

What are the 4 molecular subtypes of breast cancer? How are they determined?
- Genetic profiling can separate the 4 molecular subtypes:
1. Luminal A: ER+, Her2(-) -> better molecular subtype for tx
2. Luminal B: ER+, HER2+ -> more high-grade, so more aggressive
3. Her2+: ER-, Her2 over-expressed -> NOT good (Her2 amplification = more aggressive tumors)
4. Basal type: ER-, Her2(-) -> really difficult to tx, esp. if also progesterone (-)
What % of breast cancer is linked to inherited muts? Characterize these cancers.
- 10%
- More likely to be bilateral and to have other cancers, ovarian
- More likely to have a family history
- To develop cancer before menopause
- And belong to certain ethnic groups
- 1/3 (of this 10%) have BRCA1 or BRCA2
- Other: Li-Fraumeni (p53), Cowden (PTEN: also endometrial cancer), ataxia-telangiectasia carriers
What are BRCA1/2?
- Tumor suppressor genes -> both alleles must be inactivated
- Most carriers devo cancer by age 70 (30-90%)
- 12% of women referred for genetic testing have BRCA1/2 deleterious muts; higher in Ashkenazi Jewish women
1. Pts really need to see a genetic counselor before being tested
What is the epi of BRCA1?
- Tumor suppressor gene at 17q21 that may interact with p53; autosomal dominant (DEC penetrance)
- BRCA1 muts present in 10% of women with:
(a) No family history
(b) Breast cancer onset by age 40 years, and
(c) High grade, triple negative tumors - Breast cancer usually by ages 40-59
- Most effective predictor is age of onset < 50 years, and triple negative status (greater incidence of medullary tumors)
What is the tx and prognosis for BRCA1?
- Carriers: close F/U or prophylactic mastectomy
- Tamoxifen may prevent bilateral breast cancers in ER(-) tumors
- INC risk of recurrence after breast conserving surgery
- BRCA1 status does not appear to affect death rates, but is associated with resistance to certain chemotherapy agents
What do you see here?

-
Medullary tumor of the breast: greater incidence in BRCA1+ pts
1. If you see this on patho report, test them for BRCA1, even if no family history - Usually high grade (basal-like phenotype)
- Abundant intra- and peri-tumoral lymphocytes
- Pretty well circumscribed, with pushing border rather than infiltrative
What is the typical micro appearance of BRCA1 breast tumors?
- Usually high grade (basal-like phenotype)
- Abundant intra- and peri-tumoral lymphocytes
- Greater incidence of medullary tumors
- High incidence of DCIS

What is the epi of BRCA2?
- Tumor suppressor gene at 13q12-13
- Usually get breast cancer by age 50
- Pts also have higher risk of cancers of the ovary (39-63%), bone, pharynx, prostate, pancreas, and other organs
1. More incidence of other tumors than with BRCA1 - Slightly more frequent in black (2.6%) versus white (2.1%) American patients
What is the typical micro of BRCA2 breast tumors?
- Usually invasive ductal carcinoma, no special type
- High grade features and pushing tumor margin
- High incidence of DCIS
- Don’t have same circumscription as BRCA1 tumors
How do hormones influence breast cancer?
- Estrogen/hormone imbalance play significant role
- Long duration of reproductive life, nulliparity, and late age at birth of first child
- All INC estrogen unopposed by progesterone
- Functional ovarian tumors
What are the noninvasive (2) vs. invasive (5) breast cancers?
-
Noninvasive: still have myoepithelial cells
1. Ductal carcinoma in-situ (DCIS)
2. Lobular carcinoma in-situ (LCIS) -
Invasive
1. Invasive Ductal Carcinoma (NOS)
2. Invasive lobular carcinoma
3. Medullary Carcinoma
4. Colloid/Mucinous Carcinoma
5. Tubular Carcinoma
What are the patterns, prognosis, and treatments of DCIS?
-
Many patterns: solid, comedo, cribriform, papillary, micropapillary
1. Comedo type: central necrosis (>50%) and high grade nuclei; more likely to progress to invasion (atypical cytology) - Calcifications frequent
- Good prognosis: can take a long time for these to progress to full-blown carcinoma
- Current tx options include lumpectomy, radiation and anti-estrogenic agents like Tamoxifen
What is this?

DCIS: cribriform pattern
What is this?

- High-grade DCIS: comedo type
- These cells are huge, prominent nuclei
- Easy to pick out mitotic figures
- Pyknotic
What do you see here? Morphological keys?

- Uniform, low grade monotonous cells
- Intracellular, “targetoid” mucin
- Treatment either chemotherapy OR close F/U
- Loss of e-cadherin: come from same cell as DCIS, but this loss of e-cadherin makes it different
-
Morphological keys: tend to be much simpler cytology than DCIS
1. Look kind of like lymphos
2. Round nuclei
What is this?

LCIS
What is this?

LCIS
What is invasive ductal carcinoma?
- 70-80% of tumors fall into this category
- Can be associated with DCIS
- NO myoepithelial cell layer
- Arises from terminal duct lobular unit (as does lobular carcinoma), not ductal epithelium, so nomenclature is not actually accurate
What do you see here?

- Invasive ductal carcinoma: atypical cells
- Some ducts
- Tumor cells infiltrating into and through the stroma and collagen
- NO myoepithelial cells here (single cell layer)
What is this?

Invasive ductal carcinoma
What is this?

- Invasive ductal carcinoma with desmoplastic response
- Lighter, purple kind of stroma in the background
- High N/C ratios
What do you see?

Invasive ductal carcinoma with desmoplastic response
What is lobular carcinoma?
- Loss of expression of CDH1, gene that encodes E-cadherin -> (-) for E-cadherin, while ductal will be (+)
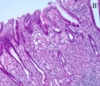
- Males and females w/heterozygous germline muts in CDH1 also have a greatly INC risk of gastric signet ring cell carcinoma (image attached)
1. Lamina propria with cells with mucin pushing nuclei out to the edge
2. Form of adenocarcinoma that produces mucin - Start to fall apart because they have lost their E-cadherin
- She said this was IMPORTANT to remember