Science Team Content Testing Flashcards
Test Deck
Testing . . .

4 . . 5. . . 6
Please define the following term:
Henry’s Law
At a constant temperature, the amount of a given gas dissolved in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid.
Pgas=kHc
P= partial pressure of the solute in the gas
kH= constant with dimensions of pressure divided by concentration.
c= concentration of the solute
Which gases diffuse in and out of blood during respiration?
- Oxygen diffuses into the blood
- Carbon dioxide diffuses out of the blood
Please identify and explain the following process depicted by the image:
The process depicted is inhalation. When one inhales:
- The diaphragm contracts and moves downward
- The rib cage expands outward
- The lungs expand due to the resulting increase in volume and decrease in pressure of the chest cavity
- Air is drawn in as the decrease in pressure falls below the atmospheric pressure
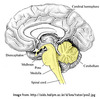
The three brain structures that regulate respiration are: 1. Midbrain, 2. Medulla Oblongata, 3. Pons
All three make up the brainstem.
Define Macrophage
A macrophage is type of white blood cell. Macrophages (Greek for “big eaters”) phagoctyose (engulf and digest) cellular debris and pathogens. They are found throughout the human body as either mobile cells that act throughout the lymphatic system or as stationary/fixed cells that act in high risk areas for microbial infection such as the alveolus of lungs, the liver, the spleen, bone, and connective tissue.
Define differential pressure.
The difference between pressure inside the lung, called intrapulmonary pressure, and pressure outside the lung, called intrapleural pressure.
How is surface tension created in the lungs?
The walls of the aveoli are coated with a thin layer of water, and the strong attraction between water molecules creates surface tension
What potential problems could this surface tension lead to?
When exhalation occurs, the area between aveoli decreases, and therefore increases the strength of the surface tension. This could prevent another expansion of the aveoli (and lungs) and prevent further breathing.
What does DNA stand for?
Deoxyribonucleic Acid
DNA is a polymer of ________, which are held together by ________ bonds.
nucleotides; phosphodiester
Label the 5’ end and the 3’ end of this single DNA strand.

The 3’ end carbon is attached to an -OH group and the 5’ end carbon is attached to a phosphate group.

Describe what is meant by the antiparallel nature of DNA.
The two strands of DNA that make up the double helix run in opposite directions, one strand with a 5’ -> 3’ orientation and the other with a 3’ -> 5’ orientation.
What are the three main parts of a nucleotide?
a phosphate group, a 5-carbon sugar, and a nitrogenous base
Name the purines and pyrimidines.
The purines are adenine and guanine. The pyrimidines are cytosine and thymine.
What is the difference in structure between purines and pyrimidines?
Purines are two ring structures while pyrimidines are single ring structures.
Which nitrogenous base forms hydrogen bonds with adenine?
thymine
Which nitrogenous base forms hydrogen bonds with guanine?
cytosine
Which type of bond forms between the nitrogenous bases of two complementary DNA strands?
hydrogen bond
How many hydrogen bonds form between adenine and thymine?
two hydrogen bonds
How many hydrogen bonds form between guanine and cytosine?
three hydrogen bonds
What is the central dogma of molecular biology?
DNA is transcribed to RNA which is translated to amino acids that make up a protein.
Define DNA helicase.
an enzyme responsible for separating the two strands of a DNA double helix needed for initiation of DNA replication
What is the name of the enzyme responsible for adding deoxyribonucleotides to a newly created DNA strand during replication?
DNA polymerase
What does DNA polymerase require to get started?
DNA polymerase cannot begin adding nucleotides by itself. An RNA primer of approximately 10 ribonucleotides is required to which DNA polymerase can begin adding nucleotides.
In what direction does DNA polymerase read the parental strand?
3’ -> 5’
In what direction is the new complementary strand created by DNA polymerase?
5’ -> 3’
What is the difference between the leading strand and the lagging strand?
The leading strand is the newly synthesized strand of DNA being replicated continuously due to the complementary parental strand’s 3’ -> 5’ direction towards the replication fork. The lagging strand is the newly synthesized strand of DNA that is replicated in a series of fragments (Okazaki fragments) due to the complementary parental strand’s 5’ -> 3’ direction towards the replication fork.
Define semiconservative in terms of DNA replication.
The semiconservative nature of replication means that when a new double helix is created, it contains one strand from the original DNA, and one newly synthesized strand.
In the Bohr Model, what does the Hydrogen electron orbit?
Nucleus
In quantum mechanics, where does the Hydrogen electron exist?
In a spherical probability cloud around the nucleus.
In quantum mechanics, what does the principle quantum number, n, define?
The principle quantum number, n, defines what shell the electron is in.
As the principle quantum number, n, increases, the energy _________.
Increases
How many electrons per orbital?
2 Electrons
How many orbitals are there per shell?
n^2
How many electrons per shell?
2n^2
Electrons are naturally found in which state?
Ground State
To which state does an electron move when it absorbs energy?
Excited State
Which is higher in energy, the excited states or the ground state?
Excited States
Energy is ______ when a electron moves from excited to ground state.
Released
Define:
Absorption Spectrum
The electromagnetic spectrum, broken by a specific pattern of dark lines or bands, observed when radiation traverses a particular absorbing medium. The absorption pattern is unique and can be used to identify the material.
Define:
Emission Spectrum
The spectrum of bright lines, bands, or continuous radiation characteristic of and determined by a specific emitting substance subjected to a specific kind of excitation.
In quantum mechanics, how many orbitals can be found in the s shell?
One orbital.
In quantum mechanics, how many orbitals can be found in the p shell?
Three orbitals.
In quantum mechanics, how many orbitals can be found in the d shell?
Five orbitals.
In quantum mechanics, how many orbitals can be found in the f shell?
Seven orbitals.
In quantum mechanics, what does l symbolize?
l is the angular momentum quantum number. l ranges from 0 to n-1.
In quantum mechanics,what do the letters s,p,d,f symbolize?
The letters s,p,d,f symbolize the subshells.
In quantum mechanics, what is the maximum number of electrons found in an orbital?
Two electrons.
In quantum mechanics, for a given shell, higher subshells have ______ energy.
Higher.
In quantum mechanics,what does the letter m signify?
The letter m signify’s the magnetic quantum number. m ranges from -1 to 1, including 0.
In quantum mechanics, what does the letter s signify?
The letter s signify’s the spin quantum number. s is either -1/2 or 1/2.
In quantum mechanics, what is the shape of the s subshell?
Spherical.
In quantum mechanics, subshells are filled by placing electrons in the _____ subshell first.
Lowest.
What are the General Characteristics of Fungi?
Fungi are eukariotic chemoheterotrophic organisms that absorb food and nutrients through chitinous cell walls. They can be either unicellular (yeast) or multicellular (mold, mushrooms) and may reproduce either sexually or asexually
What are the general functions of the mycelium of fungi?
The mycelium:
- absorbs nutrients - aids in decomposition of organic matter - is involved in fungal growth and reproduction
Describe the asexual reproductive cycle of fungi
Asexual reproduction occurs in the sporangium found on the cellular walls of reproductive hyphae cells of the mycelium of fungi. Haploid spores (n) are formed in the sporangium by mitotic divisions and then released to germinate on a suitible substrate. Fungi may also undergo budding and fragmentation.
Describe the sexual reproductive cycle of fungi
Four distinct stages:
Plasmogamy: the hyphae from two genetically distinct individuals from the same species fuse.
The heterokaryotic stage: the fused hyphae contain two haploid nuclei (n).
Karyogamy: The two haploid nuclei fuse into a diploid zygote (2n).
Meiosis and Germination: the zygote undergoes mieosis and forms haploid spores that will germinate on a suitable substrate
Viruses are considered non-living because they…
- Lack organelles and a cytoplasm
- Do not respond to external stimuli
- Do not grow by increasing in size
- Are unable to undergo metabolic functions and instead replicate using the host’s metabolic machinery
The capsid region of a virus may contain either _____ or _____ but not both.
DNA; RNA
Identify the following:

The image depicts a typical bacteriophage, with a capsid head region containing either single stranded or double stranded DNA or RNA and a protien tail region.
Compared to bacteria and animal cells viruses are _______ in size and additionally lack both a _______ and ________.
significantly smaller; cytoplasm; organelles
Define:
Bacteriophage
A bacteriophage is a virus that infects bacteria recognizable by its distinct capsid head region and protien tail.
Define:
Viral Envelope
A viral envelope is a bilayer lipid membrane that contains both viral and host protiens, encloses the capsid of some viruses, and plays a role in infection and pathogenisis.
Viruses can be classified in four general catergories based on whether they have…
DNA: ssDNA (single stranded) or dsDNA (double stranded)
RNA: ssRNA (single stranded) or dsRNA (double stranded)
A virus cannot perform its own metabolic functions, therefore it must ________ in order to reproduce.
utilize the metabolic machinery of the host cell
The six general stages in the viral life cycle are:
Adsorption
Penetration
Uncoating
Synthesis
Assembly
Release
Describe what occurs during the adsorption stage of the viral life cycle
During the adsorption stage the virus attaches itself to the membrane of a host cell. Viral attachment protiens recognize specific receptors on the outside of the cell, therefore only cells that have the appropriate receptors are suceptible to viral attack.
Identify the process occurring in the image below:

The image depicts the adsorption stage of the life cycle of an HIV virion. HIV is in the process of attaching to the CD4 receptor on the plasma membrane of a T-cell.


