5 - ECZEMA, ATOPIC DERMATITIS, AND NONINFECTIOUS IMMUNODEFICIENCY DISORDERS Flashcards
(97 cards)
What is eczema?
The word eczema seems to have originated in 543 ad and is derived from the Greek word ekzein, meaning to “to boil forth” or “to effervesce.” The term encompasses such disorders as dyshidrotic eczema and nummular eczema (NE), but at times is used synonymously for atopic dermatitis (atopic eczema). The acute stage generally presents as a red edematous plaque that may have grossly visible, small, grouped vesicles. Subacute lesions present as erythematous plaques with scale or crusting. Later, lesions may be covered by a drier scale or may become lichenified. In most eczematous reactions, severe pruritus is a prominent symptom. The degree of irritation at which itching begins (the itch threshold) is lowered by stress. Itching is often prominent at bedtime and usually results in insomnia. Heat and sweating may also provoke episodes of itching.
What is the hallmark of all eczematous eruptions?
Histologically, the hallmark of all eczematous eruptions is a serous exudate between cells of the epidermis (spongiosis), with an underlying dermal perivascular lymphoid infiltrate and exocytosis (lymphocytes present in overlying epidermis singly or in groups).
Spongiosis is generally out of proportion to the lymphoid cells in the epidermis. This is in contrast to mycosis fungoides, which demonstrates minimal spongiosis confined to the area immediately surrounding the lymphocytes.
In most eczematous processes, spongiosis is very prominent in the acute stage, where it is accompanied by minimal acanthosis or hyperkeratosis. Subacute spongiotic dermatitis demonstrates epidermal spongiosis with acanthosis and hyperkeratosis. Chronic lesions may have minimal accompanying spongiosis, but acute and chronic stages may overlap because episodes of eczematous dermatitis follow one another. Scale corresponds to foci of parakeratosis produced by the inflamed epidermis. A crust is composed of serous exudate, acute inflammatory cells, and keratin. Eczema, regardless of cause, will manifest similar histologic changes if allowed to persist chronically. These features are related to chronic rubbing or scratching and correspond clinically to lichen simplex chronicus or prurigo nodularis. Histologic features at this stage include compact hyperkeratosis, irregular acanthosis, and thickening of the collagen bundles in the papillary portion of the dermis. The dermal infiltrate at all stages is predominantly lymphoid, but an admixture of eosinophils may be noted. Neutrophils generally appear in secondarily infected lesions. Spongiosis with many intraepidermal eosinophils may be seen in the early spongiotic phase of pemphigoid, pemphigus, and incontinentia pigmenti, as well as some cases of allergic contact dermatitis.
chronic, inflammatory skin disease characterized by pruritus and a chronic course of exacerbations and remissions
Atopic dermatitis (AD)
It is associated with other atopic conditions, including food allergies, asthma, allergic rhinoconjunctivitis, eosinophilic esophagitis, and eosinophilic gastroenteritis. Because AD usually precedes the appearance of these other atopic conditions, it has been proposed that AD is the first step in an “atopic march” whereby sensitization to allergens through the skin may lead to allergic responses in the airways or digestive tract. Although this sequence of atopic conditions does occur in many children, whether the AD is causal in the development of the other manifestations of atopy is unproved but plausible. For this reason, early and effective treatment of AD is encouraged in an effort to prevent other atopic conditions. The genetic defect(s) predisposing at-risk individuals to the development of AD is the same for asthma and allergic rhinoconjunctivitis, and thus it has been difficult to prove that AD is causal in the development of other atopic conditions.
Epidemiology of Atopic dermatitis
The prevalence of AD, asthma, and allergic rhinoconjunctivitis increased dramatically in the last half of the 20th century, becoming a major health problem in many countries. The increase began first in the most developed nations, and as the standard of living has increased worldwide, so has the prevalence of AD. Rates of AD are about 30% in the most developed nations and exceed 10% in many countries, resulting in a worldwide cumulative prevalence of 20% In the most developed nations, the rates of AD plateaued in the 1990s, whereas developing nations have rates that continue to increase. Factors associated with high rates of AD are high latitude (perhaps associated with low levels of annual sun exposure) and lower mean annual temperature. A role for exposure to allergens thought to “trigger” AD is not supported by epidemiologic studies. Iceland has a very high rate of AD (27%) yet has no dust mites, few trees, and low pet ownership. However, children in Iceland often have positive skin prick tests to environmental allergens (24%). This questions the value of such tests in predicting causal environmental allergens in AD. Girls are slightly more likely to develop AD. In the United States an increased risk of AD during the first 6 months of life is noted in infants with African and Asian race/ethnicity, male gender, greater gestational age at birth, and a family history of atopy, particularly a maternal history of eczema. Other factors that increase the risk for the development of AD early in childhood include consumption of a Western diet, birth order (first children at greater risk), and delivery by cesarean section, all of which alter the intestinal microbiome. Exposure to antibiotics prenatally during the first or second and third trimesters also increases risk of AD. Therefore biodiversity in the gut microbiome seems to be protective. Gut colonization with Clostridium cluster I is associated with development of AD. Dog ownership before age 1 year decreases the risk of developing AD by age 4, but cat ownership has no effect. The hygiene hypothesis suggests that being raised on a farm lowers the risk of AD whereas living in modernized, cleaner indoor environments leads to a higher risk of AD.
About 50% of cases of AD appear in the first year of life, the vast majority within the first 5 years of life, and the remaining cases of “adult” AD usually before age 30. Atopy is now so common in the population that most individuals have a family history of atopy. Elevated immunoglobulin E (IgE) levels are not diagnostic of atopic disease in the adult. Therefore elevated IgE and a family history of “atopy” in an adult with new-onset dermatitis should not be used to confirm the diagnosis of adult AD Adult AD should only be considered when the dermatitis has a characteristic distribution and when other significant diagnoses, such as allergic contact dermatitis, photodermatitis, and cutaneous T-cell lymphoma, have been excluded. Rather, a dermatologist should infrequently make the diagnosis of adult “atopic dermatitis” for a dermatitis appearing for the first time after age 30.
Genetic Basis and Pathogenesis of atopic dermatitis
Eighty percent of identical twins show concordance for AD. A child is at increased risk of developing AD if either parent is affected. More than one quarter of offspring of atopic mothers develop AD in the first 3 months of life. If one parent is atopic, more than half the children will develop allergic symptoms by age 2. This rate rises to 79% if both parents are atopic. All of these findings strongly suggested a genetic cause for AD. Filaggrin is a protein encoded by the gene FLG, that resides in the epidermal differentiation complex (EDC) on chromosome 1q21. Filaggrin is processed by caspase 14 during terminal keratinocyte differentiation into highly hydroscopic pyrrolidone carboxylic acid and urocanic acid, collectively known as the “natural moisturizing factor” (NMF). Null mutations in FLG lead to reduction in NMF, which probably contributes to the xerosis that is almost universal in AD. Transepidermal water loss (TEWL) is increased. This may be caused by subclinical dermatitis, but also by abnormal delivery of lamellar body epidermal lipids (especially ceramide) to the interstices of the terminally differentiated keratinocytes. The resulting defective lipid bilayers retain water poorly, leading to increased TEWL and clinical xerosis. Ichthyosis vulgaris is caused by mutations in the FLG gene and is frequently associated with AD. Four FLG mutations have an estimated combined allelic frequency of 7%–10% in individuals of European descent. Different FLG gene mutations are associated with AD in other ethnicities although the rates do not necessarily match AD prevalence, demonstrating that the disease is multifactorial. Filaggrin 2 (FLG2), also in the EDC and w th similar function to FLG, is associated with persistent AD in African Americans. Inheriting one null FLG mutation slightly ncreases one’s risk of developing AD, and inheriting two mutations, either as a homozygote or a compound heterozygote, dramatically increases one’s risk. Between 42% and 79% of persons with one or more FLG null mutations will develop AD. However, 40% of carriers with FLG null mutations never have AD. FLG mutations are associated with AD that presents early in life, tends to persist into childhood and adulthood, and is associated with wheezing in infancy and with asthma. FLG mutations are also associated with allergic rhinitis and keratosis pilaris, independent of AD. Hyperlinear palms are strongly associated with FLG mutations, with a 71% positive predictive value (PPV) for marked palmar hyperlinearity. LAMA3 gene mutations, encoding the alpha chain of laminin 5, may also predispose to AD. In murine models, decreased Claudin 3 expression can lead to leakage of sweat in the superficial dermis contributing to impaired sweating in atopic dermatitis.
Not all cases of AD are associated with FLG mutations. AD patients often demonstrate immunologic features consistent with a T-helper 2 (Th2) phenotype, with elevated IgE, eosinophils on skin biopsy, and positive skin tests and radioallergosorbent test (RAST). The cytokines, especially interleukin-4 (IL-4) and interleukin-13 (IL-13), that are released due to the Th2 immune response play an important role in AD by increasing inflammatory cell infiltration and stimulating the inflammatory feedback loop. Basophils and innate type 2 lymphoid cells can also secrete IL-4 and IL-13. Thymic stromal lymphopoietin (TSLP) is an important interleukin-7 (IL-7)–like cytokine that, through its interaction with Th2 cells, basophils, mast cells, and dendritic cells, promotes the secretion and production of Th2 cytokines and the development of inflammatory Th2 CD4+ T cells (through production of OL40L). TSLP is produced by keratinocytes and is found in high levels in AD skin lesions. In addition, interleukin-31 (IL-31) is produced by Th2 and Th22 cells. IL-31 binds directly to nerves leading to itching and also downregulates expression of filaggrin. The JAKSTAT pathway is also critical in causing overactivation of the TH2 response and itch. Interleukin-17 (IL-17) and interleukin-22 (IL-22), which are released by Th17 cells, are also elevated in AD. Thus AD appears to represent a disorder characterized by a barrier defect that activates a specific Th2 response leading to a cycle of inflammation mediated by various inflammatory signals. The cytokines produced then worsen the already defective barrier. This leads to a vicious cycle of barrier failure and progressive inflammation, producing a chronic, relapsing, pruritic disorder, and explains why moisturization alone is not enough once the inflammatory cascade has started.
Preventionof AD in High-Risk Children
Extensive studies have been undertaken to determine whether it is possible to prevent the development of AD in children at high risk—those with parents or siblings with atopy. The most promising studies now repeated multiple times show that early moisturization, starting before 3 weeks of life, with thick emollient, may prevent AD in children at high risk of developing AD. Twice daily moisturization with various emollients such as petrolatum, sunflower seed oil, and others leads to an approximately 50% reduction in the expected rate of AD development in the first 6 months of life. This supports the idea that breaks in the skin barrier are an essential step in developing AD.
Switching to soy formula does not appear to reduce the risk of developing AD. Prolonged exclusive breastfeeding beyond 3–4 months of age is not protective for the development of AD. Extensively hydrolyzed casein formulas may be used as a supplement or substitute for breast milk during the first 4 months of life. Maternal allergen avoidance during pregnancy does not reduce the risk of AD in the offspring. Probiotic administration during and after pregnancy has been shown to decrease AD incidence by 14% based on data from a meta-analysis However, the type of probiotic to use, exactly when to start, and the safety during pregnancy are not fully elucidated. House dust mite (HDM) avoidance does not reduce AD, even in sensitized individuals, and high levels of HDMs in the environment in early life reduce AD risk.
Role of Food allergy in AD
The role of food allergy in AD is complicated, and the purported role of foods in AD has changed in recent years. Approximately 35% of children with moderate to severe AD have food allergies. However, 85% of children with AD will have elevated IgE to food or inhalant allergens, making a diagnosis of food allergy with serum or prick tests alone challenging due to the high false-positive rate of testing. Before food allergy testing is undertaken, treatment of the AD should be optimized. Parents are often seeking a “cause” for the child’s AD, when in fact it could be controlled with appropriate topical measures. Food restriction diets can be difficult and could put the child at risk for malnourishment and possibly development of allergy and should never be pursued without the oversight of an allergist and nutritionist to ensure proper nutrition. Food allergy should be pursued only in younger children with moderate to severe AD in whom standard treatments have failed. These children should also have a history of possible triggering of AD by specific food exposures. Testing, if performed, should be targeted foods to which the child is likely to be exposed, but generally wheat, egg, soy, cow’s milk, and peanut testing has been the most relevant to the AD. Double-blind placebo-controlled food challenges are
the “gold standard” for diagnosing food allergy. Skin prick tests have a high negative predictive value (>95%) but a PPV of only 0%–65%. For example, more than 8% of the U.S. population has a positive prick test to peanut, but only 0.4% are actually clinically allergic. Possible food allergy detected by testing should be confirmed by clinical history A positive RAST or skin prick test for a food that the child rarely or never ingests is probably not causally relevant to the child’s AD. Higher serum IgE levels and larger wheal sizes (>8–10 mm) are associated with greater likelihood of reacting to these foods when challenged. About 90% of food allergy is caused by a limited number of foods, as follows:
- Infants: cow’s milk, egg, soybean, wheat
- Children (2–10 years): cow’s milk, egg, peanut, tree nuts, fish, crustacean shellfish, sesame, kiwi fruit
- Older children: peanut, tree nuts, fish, shellfish, sesame, pollenassociated foods
Breastfeeding mothers must avoid the incriminated foods if their infant has been diagnosed with a food allergy.
There has been a rapid rise in peanut allergy in the United States. Recommendations to limit peanut exposure in childhood has been revised. A large randomized placebo control trial showed that early exposure in children at high risk for peanut allergy (due to strong family history of atopy) decreased the rate of development of peanut allergy by 86%. Therefore the revised guidelines that were developed in conjunction with dermatologists and allergists for peanut exposure are based on AD in the child. Of course a whole peanut should never be given to an infant due to choking hazard; therefore this was done with smooth peanut butter or the peanut snack Bamba (trademark).
1 Children with severe AD or egg allergy should be considered for peanut testing before exposure to peanuts around 4–6 months of age.
- Mild to moderate AD: Introduce peanuts around 6 months.
- No AD or food allergy: Introduce peanuts in accordance with family and cultural preferences.
Role of aeroallergens in AD
It is debated how much of a role aeroallergens play in the pathophysiology of AD. Although early exposure to allergens may lessen the incidence of AD, exposure to dust mites, animal allergens, mold, pollen, tree allergens, and other airborne allergens in those who are allergic may exacerbate AD. Testing for IgE responses to aeroallergens may not be predictive of their effect on patients with AD. In patients who note worsening around specific allergens, they should avoid these. Common allergens to avoid if possible include tobacco smoke, pollen, house dust mites, cats, and dogs (if allergic) although early exposure to dogs may prevent AD. Reduction of dust mite exposure can be achieved through vacuuming with a filtered vacuum, using dust mite covers on mattresses, avoidance of stuffed animals, and wall to-wall carpeting if feasible.
3 stages of AD
AD can be divided into three stages:
- infantile AD, occurring from 2 months to 2 years of age;
- childhood AD, from 2–10 years; and
- adolescent/adult AD.
What are the clinical manifestations of AD?
In all stages, pruritus is the hallmark. Itching often precedes the appearance of lesions, thus the concept that AD is “the itch that rashes.” Useful diagnostic criteria include those of Hannifin and Rajka, the UK Working Party, and the American Academy of Dermatology’s Consensus Conference on Pediatric Atopic Dermatitis (Boxes 5 1 and 5.2). These criteria have specificity at or above 90% but have much lower sensitivities (40%–100%). Therefore these criteria are useful for enrolling patients in studies and ensuring that they have AD, but less practical in diagnosing a specific patient with AD.
Hannifin and Rajka Criteria for Atopic Dermatitis

Modified Criteria for Children With Atopic Dermatitis

Infantile Atopic Dermatitis

Fifty percent or more of AD cases present in the first year of life, but usually not until after 2 months. Widespread dermatitis in children under 2 months may be from an irritant, ichthyosis, or a hallmark of severe immunodeficiency. AD in infancy usually begins as erythema and scaling of the cheeks (Fig. 5.1). The eruption may extend to the scalp, neck, forehead, wrists, extensor extremities, and buttocks. There can be overlap with seborrheic dermatitis on the scalp and in the folds, but papular or nodular involvement in the ax llae and inguinal folds is more typical of scabies infestation. Children with AD who have FLG gene mutations specifically have more cheek and extensor arm/hand involvement. There may be significant exudate; secondary effects from scratching, rubbing, and infection include crusts, infiltra ion, and pustules, respectively. Occlusion of saliva due to teething, extensive breastfeeding, and drooling may cause the cheeks and upper chest to flare and thick emollients can help prevent this. Breastfeeding should not be curtailed. The infiltrated plaques eventually take on a characteristic lichenified appearance The infantile pattern of AD usually disappears by the end of the second year of life.
Worsening of AD is often observed in infants after immunizations and viral infections likely due to activation of the immune system. Partial remission may occur during the summer, with relapse in winter. This may relate to the therapeutic effects of ultrav olet (UV) B light and humidity in many atopic patients, as well as the aggravation by wool and dry air in the winter. Extensive airborne environmental allergies may lead to worsening in the warmer months.
Childhood Atopic Dermatitis
Fig. 5.2 Flexural involvement in childhood atopic dermatitis.

During childhood, lesions tend to be less exudative. The classic locations are the antecubital and popliteal fossae (Fig. 5.2), flexor wrists, ankles, eyelids, face, and around the neck. Lesions are often lichenified, indurated plaques. These are intermingled with isolated, excoriated, 2–4 mm papules that are scattered more widely over the uncovered parts. Nummular morphology and involvement of the feet are more common in childhood AD.
Pruritus is a constant feature, and most of the cutaneous changes are secondary to it. Itching is paroxysmal. Scratching induces lichenification and may lead to secondary infection. A vicious cycle may be established, the itch-scratch cycle, as pruritus leads o scratching, and scratching causes secondary changes that in them cause itching. Instead of scratching causing pain, in the atopic patient the “pain” induced by scratching is perceived as itch and induces more scratching. The scratching impulse is beyond the control of the patient. Severe bouts of scratching occur during
sleep, leading to poor rest and chronic tiredness in atopic children. This can affect school performance. Parents often scold children who are scratching, and this leads to more anxiety and thus more scratching.
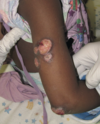
Severe AD involving a large percentage of the body surface area BSA) can be associated with growth retardation (Fig. 5.3). Restriction diets and steroid use may exacerbate growth impairment. Aggressive management of such children with phototherapy or systemic immunosuppressive agents may allow for rebound growth. Children with severe AD may also have substantial psychological disturbances. Parents should be questioned with regard to school performance and socialization. Although using light therapy and systemic immunosuppressive or immunomodulatory therapy in children can be daunting for patients and practitioners, the benefits to quality of life can dramatically outweigh the risks.
Fig. 5.3 Severe, widespread atopic dermatitis.

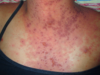
Atopic Dermatitis in Adolescents and Adults

Fig. 5.4 Prurigo-like papules in adult atopic dermatitis.
Most adolescents and adults with AD will give a history of childhood disease. AD will begin after age 18 years in only 6%–14% of patients diagnosed with AD. One exception is the patient who moves from a humid, tropical region to a more temperate area of higher latitude. This climatic change is often associated with the appearance of AD. In older patients, AD may occur as localized erythematous, scaly, papular, exudative, or lichenified plaques. In adolescents, the eruption often involves the classic antecubital and popliteal fossae, front and sides of the neck, forehead, and area around the eyes. In older adults, the distribution is generally less characteristic, and localized dermatitis may be the predominant feature, especially hand, nipple, or eyelid eczema. At times, the eruption may generalize, with accentuation in the flexures. The skin generally is dry and somewhat erythematous. Lichenification and prurigo-like papules are common (Fig. 5.4). Papular lesions tend to be dry, slightly elevated, and flat topped. They are almost always excoriated and often coalesce to form plaques. Staphylococcal colonization is common. In darker-skinned patients, the lesions are often hyperpigmented, frequently with focal hypopigmented areas related to healed excoriations.
Itching usually occurs in crises or paroxysms. Adults frequently complain that flares of AD are triggered by acute emotional upsets. Stress, anxiety, and depression reduce the threshold at which itch is perceived and result in damage to the epidermal permeability barrier, further exacerbating AD. Atopic persons may sweat poorly and may complain of severe pruritus related to heat or exercise. Physical conditioning and liberal use of emollients improve this component, and atopic patients can participate in competitive sports.
Even in patients with AD in adolescence or early adulthood, improvement usually occurs over time, and dermatitis is uncommon after middle life. In general, these patients retain mild stigmata of the disease, such as dry skin, easy skin irritation, and itching in response to heat and perspiration. They remain susceptible to a flare of their disease when exposed to a specific allergen or environmental situation. Photosensitivity develops in approximately 3% of AD patients and may manifest as either a polymorphous light eruption–type reaction or simply exacerbation of the AD by UV exposure. The average age for photosensitive AD is the middle to late thirties. Human immunodeficiency virus (HIV) infection can also serve as a trigger, and new-onset AD in an at-risk adult should lead to counseling and testing for HIV if warranted.
The hands, including the wrists, are frequently involved in adults, and hand dermatitis is a common problem for adults with a history of AD. It is common for irritant or atopic hand dermatitis to appear in young women after the birth of a child, when increased exposure to soaps and water triggers their disease. There is a new trend in children who can buy kits or mix their own various household liquids such as glue, borax, contact solution, baking soda and others to make “slime” This can lead to irritant dermatitis or worsening of hand atopic dermatitis. Wet work is a major factor in hand eczema in general, including those patients with AD.
Fig. 5.5 Atopic hand dermatitis.

Atopic hand dermatitis can affect both the dorsal and the palmar surface (Fig. 5.5). Keratosis punctata of the creases, a disorder seen almost exclusively in black persons, is also more common in atopic patients. Patients with AD have frequent exposure to preservatives and other potential allergens in the creams and lotions that are continually applied to their skin. Contact allergy may manifest as chronic hand eczema. Patch testing can help differentiate an allergic contact dermatitis.
Fig. 5.6 Periocular atopic dermatitis.

Eyelids are often involved (Fig. 5.6). In general, the involvement is bilateral and the condition flares with cold weather. As in hand dermatitis, irritants and allergic contact allergens must be excluded by a careful history and patch testing.
linear transverse fold just below the edge of the lower eyelids
Dennie-Morgan fold - indicative of the atopic diathesis, although it may be seen with any chronic dermatitis of the lower lids
In atopic patients with eyelid dermatitis, increased folds and darkening under the eyes is common.

When there is extensive facial involvement, the nose is still typically spared. This is called the ______
“headlight sign.”
The axillary vault and inguinal folds are also typically spared likely due to high humidity in these areas.

A prominent nasal crease may also be noted due to chronic upward wiping of the nose when there is rhinitis secondary to seasonal allergies. This is called the _____
“nasal salute.”
Other manifestations of AD
The less involved skin of atopic patients is frequently dry and slightly erythematous and may be scaly. Histologically, the apparently normal skin of atopic patients is frequently inflamed subclinically. The dry, scaling skin of AD may represent low-grade dermatitis. Pityriasis alba is a form of subclinical dermatitis, frequently atopic in origin. It presents as poorly marginated, hypopigmented, slightly scaly patches on the cheeks, upper arms, and trunk, typically in children and young adults with types III to V skin. It usually responds to emollients and mild topical steroids, preferably in an ointment base.
consists of horny follicular lesions of the outer aspects of the upper arms, legs, cheeks, and buttocks and is often associated with AD, AD and occurs in patients with filagrin mutations.

Keratosis pilaris (KP)
The keratotic papules on the face may be on a red background, a variant of KP called keratosis pilaris rubra faceii. KP is often refractory to treatment. Moisturizers alone are only partially beneficial. Some patients will respond to topical lactic acid, urea, or retinoids but they can easily irritate the skin of atopic patients and should be avoided in young children. If older patients desire, treatment should begin with applications only once or twice a week. KP must be differentiated from follicular eczema which tends to affect the trunk and is often more prominent in patients with skin ypes III-VI.
Thinning of the lateral eyebrows is sometimes present in AD

Hertoghe sign
This apparently occurs from chronic rubbing caused by pruritus and subclinical dermatitis. Hyperkeratos s and hyperpigmentation, which produce a “dirty neck” appearance, are also common in AD patients.
blanching of the skin at the site of stroking or scratching
White dermatographism
Chronic exposure to the vasoconstrictive effects of topical and oral steroids may lead to erythroderma due to vasodilation when steroids are tapered. This can lead to dysesthesias and belies the importance of using maintenance therapies that limit steroid exposure or lessen strength.
Atopic patients are at increased risk of developing various forms of urticaria, including contact urticaria. Episodes of contact urticaria may be followed by typical eczematous lesions at the affected site because skin that is scratched may flare with AD.