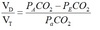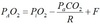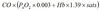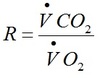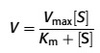Equations Flashcards
Fick’s Law
The net rate of diffusion is proportional to the diffusion co-efficient (1/ square root of MW) , surface area, concentration gradient and inversely dependant on thickness of the boundary
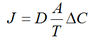
Nernst Equation
Equlilibrium potential – the membrane potential at which electrical and chemical gradient of individual ions are equal. For cations, o/i. For anions i/o.
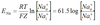
Goodman equation
The membrane potential depends on the distribution of and the membrane permeability to Na, K, Cl.
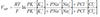
Pouseuille’s law
n = viscosity

Reynolds number
Re >2000 is turbulent
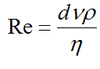
Henderson
At 37°, Ka x 0.03 = 24
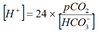
Henderson Hasselbalch
pKa=6.1 at 37°
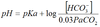
Standard deviation
SD = √variance.
Measure of dispersion or spread of a normal distribution.
95% of data points lie within 1.96 SD of the mean.
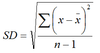
Chi square
Difference in observed from expected in nominal data, based
on contingency table. Compares rates or proportions.

Shunt equation
Calculated to give estimate of venous admixture – gives ‘virtual shunt’, the amount of shunt which would be present if the shunt was entirely of mixed venous blood.
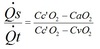
Laplace’s law
T=surface tension
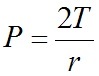
Force
Force = Pressure X Area
Pressure = F/A
Work
Work = force x distance
Work of breathing
The pressure volume characteristics also determine
work of breathing.
Bohr equation
Physiological dead space.
PECO2 = mixed expired CO2. Use PaCO2 for PACO2.
Normally