7.06 Drugs affecting the Hypothalamic-Pituitary Axis Flashcards
(40 cards)
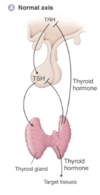
Describe the normal growth hormone axis
- GHRH is synthesised and secreted from the hypothalamus
- This stimulates GH release from the anterior pituitary
- This acts in the liver to cause IGF release to act on target tissues
- Somatostatin from the hypothalamus inhibits the release of GHRH
- Ghrelin also promotes
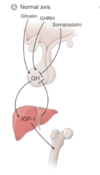
What would the axis look like if there were growth hormone insensitivity?
Insensitivity means that the liver is not responsive to GH levels and the lack of negative feedback increases the levels of GH release with no impact on rising IGF
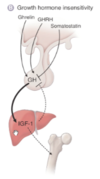
What would secondary and tertiary deficiencies in growth factor look like?
- Secondary means that there is a lack in the GH itself
- Tertiary means there is a lack of GHRH
Why does growth hormone (somatostatin) have zero bioavailability after oral administration?
Because it is a protein (nutrient) so oral administration leaves it prone to enzymatic degradation in the mouth and gut. So therapeutically it requires parenteral (through an injection)
What is another consequence of the protein nature of Somatostatin?
- Short half-life
- Daily or multi-daily
Requires: titration of dose to effect: replacing a physiologically regulated hormone.
There is lots of interaction between endocrine system… How does Growth hormone impact thyroxine levels?
GH can cause reduced T4 levels
Why is it important to titrate the doses of growth hormone?
- Too much = develop acromegaly
- Too little = short of stature or will have different deficiencies in adults
Growth hormone also has trophic effects and has a long acting effect - thus need to monitor the releasing hormone
How do the Ghrelin and GHRH receptors respond to cause production and release of GH
GHRH receptor: elevates cAMP
Ghrelin receptor: elevates Ca2+
What evidence shows that a combination of Ghrelin and GHRH elicits a larger release of GH synergistically
Because of the two different mechanisms achieving the same goal, there is scope for large amplification when combined
Ghrelin is under investigation as a therapy for GH deficiency (and other disorders, mostly eating-related)

How can IGF-1 (insulin-like growth factor 1) be used pharmaceutically?
Useful for GH-insensitivity (called Laron dwarfism) and patients with anti-GH antibodies
What are the side effects of IGF-1?
Sometimes hypoglycaemia due the insulin like effects of IGF-1
What does too much growth hormone manifest as?
Acromegaly
Elongation and growth of structures with lots of cartilage continue growing especially the jaw, nose, hands and feet. Can lead to arthritis and other consequences
What are some therapeutic options for increased growth hormone?
- Remove tumor (if relevant)
- Reduce GH release: somatostatin analogues and dopamine agonist
- Inhibit GH action: GH antagonist, pegvisomant
How can we localize the GH secreting tumours?
Find the tumour by exploiting receptor kinetics to localise GH receptor expressing tumours
This is because the GH receptor in these gets internalised when bound to a ligand GH
Somatostatin can be used to reduce GH release (the more somatostatin, the more inhibition on the anterior pituitary to produce GH)
Describe somatostatin in terms of pharmacokinetics
It has poor pharmacokinetics
- Somatostatin reduces GH release from the pituitary (but can also reduce TSH)
- Tumor cells expressing receptors for somatostatin usually respond to somatostatin with reduced secretion of GH
- Somatostatin has a very short half-life due to enzymatic cleavage and renal elimination (it is also a protein)
What is a major strategy to confer resistance to enzymatic cleavage for somatostatin (and other proteins) for use therapeutically?
The native form of the proteins is made up of L-amino acids (the natural kind). Changing the structure of proteins by replacing some of the L-amino acids to D-amino acids make the peptides fit less well in proteolytic enzymes
These analogues give longer half lives (but they still have renal degradation)

What two additional methods can be used to better the pharmacokinetics of peptides?
- Depot formulations also exist
- Addition of dopamine agonists (bromocriptine or cabergoline) my increase effectiveness
Describe growth hormone antagonists
It has been found that a Glycine at amino acid position 119 is essential for GH to have its action as a growth hormone. Mutant GH without this amino acid (replaced) causes a loss in agonist activity.
Describe the normal binding of growth hormone to the growth hormone receptor.
The growth hormone receptor is part of the tyrosine kinase family receptor: Binding of growth hormone causes receptors to dimerises and then have the intracellular signalling effects.
Thus growth hormones have 2 binding sites motifs.
- Binding site 1 has high affinity
- Binding site 2 leads to receptor activation
How do the growth hormone antagonists interfere the normal binding and activation of GH receptors?
Mutant GH usually has a removal of the glycine and replacement with something else. This removes binding site 2 meaning binding is enabled (competition) but activation is not enabled (antagonist).
It is still a peptide so still has a short half-life
What is PEGylation?
How is it useful in pharmacokinetics?
Hanging a polyethylene glycol (PEG) –Carbon chains with O2 scattered through them
- Makes molecules larger (prevents renal filtration)
- It makes it hard for proteolytic enzymes from reaching the protein core – increases steric hindrance
- Makes them more soluble (longer half-life)

Where in the GH peptide would PEGylation occur?
What is the problem encountered by this?
PEGylation occurs at the lysines.
This is a problem because there are two lysines involved in site 1 binding, this reduces the affinity of the binding of the mutant GH
What technique is used to counteract the reduced affinity of binding coming from the PEGylation of lysines?
Modification other amino acids in the peptide to compensate for the lysine.
There is still a short half life
Describe the drug Pegvisomant
A growth hormone antagonist with the glycine replaced (antagonist activity), PEGylated with the lysine replaced.
It is a trade off between a reduced affinity somewhat (tolerable), not enough to completely reduce use