Lec 4- Antibody therapies Flashcards
1
Q
What are antibodies
A
- Antibodies (also known as immunoglobulins) are large, complex protein macromolecules
- They are produced by the adaptive immune systems to recognise and specifically target foreign materials, known as Ag
- Ab’s are produced by B cells
2
Q
Types of Antibodies
A
- Antibodies generally comprise of the following structural elements
- 2 large heavy polypeptide chains (50,000 molecular weight)
- 2 small light chains (23,000 MW)
- There are several classes (isotypes) of Ab’s produced in the human body
- 5 main types: IgG, A, M, E, D
- There are classed based on their heavy chain and perform different roles
3
Q
Antibodies Isotypes
A
- Of these, IgG is the most abundant and most of the currently approved anti-body based drugs are of the IgG class
4
Q
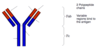
Diagnostic testing of your knowledge: Figure of IgG Ab
A
- The lower portion of Y is the heavy chain
- Arms make the light chain
- Tips are the variable region which gives the Ig Ab specificity for Ag binding
5
Q
Antibody structure
A
- These chains are held together by non-covalent binding and disulfide bonds
- The specificity of a given Ab is determined by the amino acid sequence located at the end of it’s Fab region
- By employing cloned immune cells to obtain antibodies, single specificity antibodies referred to as mAb can be produced
6
Q
What are polyclonal Ab preparations
A
- Polyclonal Abs are derived from different B cell lines
- They are a mixture of Ab’s secreted against a specific Ag, each recognising a different epitope
7
Q
Polyclonals
A
- Maybe categorized into groups based on their target specificities
- Ab’s raised against
- Specific microbial or viral pathogens
- Micro toxins
- Snake/spider venom (Anti-venoms)
- Ab’s raised against
8
Q
Polyclonal Ab’s
A
- Antibodies are produced by immunising healthy animals (e.g. horse) or humans with appropriate antigen. Blood is harvested and the antibodies extracted

9
Q
Monoclonal Antibodies (mAbs)
A
- mAbs are the most widely used form of cancer immunotherapy at this time
10
Q
Development of antibody-based therapies
A
- The main concerns are
- Developing mAb’s of appropriate specificity and affinity
- Engaging an appropriate mechanism (Drug action and tagging)
- Suitable PK
- Avoiding host immune response
11
Q
Murine, chimeric or humanised Abs
A
- First mAbs were made entirely of mouse cells
- Problems: Immune response against them
- Allergic-type reactions
- Effective first time, but after that they are destroyed
12
Q
Murine mAbs
A
- Key advances in the production of monoclonal antibodies have supported their increased application including the improved ability to produce appropriate quantities of pure antibodies and more importantly the ability to prepare humanized antibodies
- Prior to this, the use of murine based Ab therapeutics (e.g. OKT-3, muromonab-CD3, approved for use within the US) resulted in antibody therapies generally being limited to acute, single treatments such as tissue rejection after a kidney transplant
13
Q
Developments
A
- The use of murine antibodies often results in the patient’s immune system inducing human anti-mouse Ab’s that can lead to lack of efficacy, rapid clearance of the Ab, adverse reactions to the antibody and often reduced the efficacy
- Recombinant technologies have supported the production of less immunogenic mAbs
- Chimeric Ab’s are composed of murine variable regions fused with human constant regions E.g. rituximab
- Humanised Ab’s are produced by grafting murine hypervariable amino acid domains into human antibodies E.g. trastuzumab
14
Q
Human mAb’s
A
- Complete human mAb constructs can now also be produced due to developments in phage display technology and transgenic mic
- E.g. adalimumab
15
Q
Chimeric or humanized
A
- Depends on how much of the mAb is human
- The more human the more likely they will be safe




