Clinical Trials Flashcards
What is a clinical trial?
Any form of planned experiment which involves patients and is designed to elicidate the most appropriate method of treatment for future patients with a medical condition
What are the 2 main purposes of a clinical trial?
To provide reliable evidence of treatment efficacy & safety
Efficacy: ability of intervention to improve the health of a defined group under specific conditions
Safety: ability of intervention not to harm a defined group under specific conditions
What 3 things must a clinical trial be in order to give a fair comparison of effect and safety?
- Reproducible - in experiemental conditions
- Controlled - comparison of interventions to show it is the intervention that’s made the difference
- Fair - unbiased without confounding
What is the difference between efficacy and effectiveness?
Efficacy = ideal clinical conditions
Effectiveness = real world clinical experience
What are the 4 different phases of a clinical trial?

What are non-randomised clinical trials?
Clinical trials involving the allocation of patients recieving a new treatment to compare with a group of patients recieving the standard treatment
What are the disadvantages of non-randomised clinical trials?
Allocation bias - by patient, clinician or investigator
Confounding - known and unknown
What are comparison with historical controls?
Comparison of a group of patients who had the standard treatment with a group of patients recieving new treatment
What are the disadvantages of using comparison of historical controls for the ‘standard treatment’ group?
- Selection less rigorous
- treated differently from ‘new treatment’ group
- less information about bias/ confounders
- unable to control for confounders
Is this observed rate ratio statistically significant? Explain why
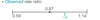
NO
The observed rate ratio and confidence interval crosses 1 therefore an affect may be due to chance
1= no effect at all
For what reasons do you need to pre-define trial outcomes at the start of the trial?
- prevent ‘data dredgind’, ‘repeated analyses’
- protocol for data collection
- agree criteris for measurement and assessment of outcomes
What is the difference between primary and secondary outcomes?
Primary Outcomes:
- Only one primary outcome
- Used in the sample size calculation
Secondary Outcomes:
- other outcomes of interest
- often includens occurence of side effects
What are the 3 different types of trial outcome?
Patho-physiological
- tumour size, thyroixine levels, ECG changes
Clinically defined
- death (mortality)
- disease (morbidity)
- disability
Patient-focused
- QoL
- psychological well-defined
- social well being
- satisfaction
What are the features of an ideal trial outcome?
- Appropriate and Relevant
- Valid and attributable (linked to the treatment compared)
- Sensitive and Specific
- Reliable and Robust
- Simple and Sustainable
- Cheap and Timely
How are measurements timed throughout a clinical trial?
- Baseline measurement taken of relevant factors
- Monitoring during the trial
- for possible effect
- for adverse effects
- Final measurement of outcomes


