9. Lipids Flashcards
(23 cards)
Lipids, or fats, are full of what?
Flavour and energy.
Lipids are also not strictly polymers, in that they are not made of only repeating monomer units. We consider them to be what?
Macromolecules.
Those of you familiar with your organic chemistry may recognise the similarities between triglycerides and what?
Esters.
An alcohol linked to a carboxylic acid makes an ester. A triglyceride, then, is simply a triester, tri- referring to its three what?
Ester linkages.
The ester linkage is the functional group of a triglyceride, and is presented as −COO−, where there is a C=O and a C−O bond.
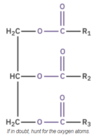
We classify triglycerides based on whether their fatty acids are saturated or unsaturated.
A fatty acid is saturated when it is made of only what?
C−C single bonds.
A fatty acid is unsaturated when there are C=C double bonds or what?
C≡C triple bonds.
Saturated fatty acids are typically linear and tightly packed, whereas unsaturated fatty acids are kinked and do not pack as easily. Saturation affects a triglyceride’s physical properties, as we shall see in the next section.
We call unsaturated fatty acids what where they only possess one double or triple bond, and polyunsaturated when there is more than one of these bonds.
Monounsaturated.

Generally, lipids are soluble in non-polar solvents, such as benzene, and insoluble in polar solvents, like what?
Water.
They are also generally colourless, odourless and tasteless.
However, a lipid’s consistency varies per molecule; some lipids are present as solid and others as liquid. Triglycerides are no exception, and we will continue our study by looking at fats and oils.
Depending on the triglyceride, it can appear as what?
Solid fat or as liquid oil.
Whether a triglyceride is solid or liquid depends on what?
The saturation of its fatty acids.
Saturated fatty acids, with their linear chains and single bonds, pack together tightly. What is an example?
Animal fat and butterfat are both triglycerides with high amounts of saturated fatty acids. These tightly packed molecules have strong intermolecular forces requiring more energy to be overcome, so their melting point is high.
unsaturated fatty acids, with their kinked chains and double or triple bonds, pack together loosely. What is an example?
Vegetable oils like sunflower or canola oil are both primarily composed of unsaturated fatty acids. These loosely packed molecules have weaker intermolecular forces requiring less energy to overcome, so their melting point is low.
What fat is a triglyceride somewhere between saturated and unsaturated fat?
Trans fat.
Food manufacturers found that by hydrogenating (adding hydrogen to) unsaturated fats (namely cheap vegetable oils), they could produce a shelf-stable solid. These solids are trans fats, which although still unsaturated, are closer to saturated fats in consistency and taste—at a fraction of the cost.
The reason trans fats are called “trans” is because the carbon atoms around the double bond lie on opposite sides. In contrast, most naturally occurring unsaturated fatty acids exist in the cis configuration, where the carbon atoms lie on the same side.
The cis configuration is what produces the kink in unsaturated fatty acids, responsible for their what?
Loose packing and weak intermolecular forces. The trans configuration of trans fats helps to straighten out the fatty acid chains. This means they can pack more closely together, similarly to saturated fatty acids.

Triglycerides are also involved in many different reactions.
They can be broken into glycerol and free fatty acids in two ways. What are they?
One, hydrolysis, simply requires water and an acid catalyst. The other, digestion, requires enzymes such as lipase. Digesting triglycerides is important for the human body, as the fatty acids can be oxidised into energy.
Another reaction involving triglycerides is saponification (soap formation) using what?
Lye, a strong base.
When triglycerides react with strong bases, they also separate into glycerol and free fatty acids. The fatty acids combine with the metal ion in lye to form a carboxylate salt, the soap itself. This crude soap is purified, scented and/or medicated to produce the variety of soaps we see today.
Lye is the common term for what?
Metal hydroxides, such as sodium or potassium hydroxide.
Triglycerides form the fat in our body, storing excess energy from what?
Digested fat, carbohydrates or protein.
How is fat used by the body?
Fat can be oxidised to release energy.
On the body, fat is a good thermal insulator (think of the brown bear or the hoiho, the yellow-eyed penguin) and a good cushion, implicated to be protective against fall fractures in the elderly.
Why are fats important for the body?
Some triglycerides or fatty acids are essential because the human body cannot synthesise them, meaning we can only obtain them from our diet. Some examples include omega-3 and omega-6 fatty acids, and having a balanced ratio of the two can reduce cholesterol and fight inflammation.
Additionally, the vitamins A, D, E and K are fat-soluble and require fat in order to be adequately absorbed.
The type of fat consumed can affect your body’s biochemistry. Which fats are bad for you?
Saturated fats have been proven to raise the levels of ‘bad’ LDL (low density lipoprotein) cholesterol and lower the levels of ‘good’ HDL (high density lipoprotein) cholesterol.
Which fats are considered healthy fats?
Unsaturated fats are considered healthy fats as they do not alter the LDL:HDL ratio.
This is why you often see recommendations from health providers to replace your saturated fat sources with sources of unsaturated fats.
What are 3 lipids outside of triglycerides?



