Sulfa, Quinolones Etc Flashcards
Sulfonamides MOA
block various steps in the purine metabolism pathway, thus inhibiting the production of nucleic acids and DNA
Significance for PABA in bacteria
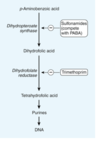
Many bacteria, unlike mammals, cannot use folate from their environment but must synthesize it from PABA
Relationship between PABA and sulfonamides
As structural analogs of PABA, sulfonamides inhibit dihydropteroate synthase and folate production via competitive inhibition.
Combination of a sulfonamide with an inhibitor of dihydrofolate reductase (trimethoprim or pyrimethamine) provides synergistic activity because of sequential inhibition of folate synthesis.
Mechanism of resistance against sulfonamides
Lack of target: some species of bacteria use exogenous folate and do not have this pathway
Mutated enzyme that drug can’t bind
Toxicities of sulfa drugs
These drugs are all contraindicated in newborns and nursing mothers as they displace unconjugated bilirubin from albumin, increasing the risk of kernicterus.
They can all cause bone marrow suppression that may or may not be due to folate underproduction: nutritional vs. immune-mediated BM suppression, megaloblastic anemia
Sulfonamides increase oxidative stress and thus can precipitate hemolytic anemia in G6PD-deficient patients. This will look like a typical hemolytic crisis you should be familiar with from last year: sudden jaundice, dark urine, normocytic anemia, elevated reticulocyte count.
Solubility of the drugs is a major problem, which can result in crystallization in urinary tract when patients become dehydrated and their urine is more concentrated; treatment is to hydrate and give IV bicarbonate as they are more soluble in alkaline fluids
Rashes are tremendously common with sulfa drugs and can range from photosensitivity, annoying-to-fatal skin rashes like Stevens-Johnson syndrome
Sulfa drugs are very allergenic and can cause hypersensitivity reactions that look like autoimmune disorders: arthritis, conjunctivitis, stomatitis, polyarteritis nodosa, psychosis
Since these hypersensitivity drug reactions are not unique to sulfa drugs, the way they will usually be asked about on exams is to have the patient on a sulfa drug, and then a specific question will be asked about diagnosing the reaction rather than the drug itself.
Examples of dihydrofolate reductase inhibitors
Trimethoprim (TMP) and pyrimethamine
Use of dihydrofolate inhibitors
Commonly be complexed to sulfonamides for synergistic and sequential inhibition of the pathway. TMP is used in bacterial disease complexed as TMP/SMZ, and pyrimethamine for protozoal (parasitic) diseases.
Toxicities of dihydrofolate inhibitors and population most susceptible to them
Toxicity for these drugs is similar to the sulfonamides and increased in AIDS patients, who commonly take these drugs daily for prophylaxis or treatment of opportunistic infections.
Folate DNA pathway
MOA of fluroquinolones
Fluoroquinolones inhibit the DNA gyrase enzyme complex.
This complex has multiple subunit enzymes, and the drugs specifically inhibit:
Topoisomerase II, preventing uncoiling of DNA for transcription and replication, and
Topoisomerase IV, preventing separation of duplicated chromosomes into daughter cells.
First line use of fluroquinolones
Earlier generations of FQs have gram - coverage only, later generations add gram + coverage.
Examples and uses of gen 2-4 of fluroquinolones
- Ciprofloxacin–“urinary FQ”, includes Pseudomonas aeruginosa coverage
- Levofloxacin–“respiratory FQ”
- Moxifloxacin–very broad coverage for resistant pathogens in hospitalized patients
How is fluroquinolones resistance achieved
Low-level plasmid-mediated: Qnr chaperone proteins that protect DNA gyrase. These give a permissive effect so that the bacteria can develop higher-level resistance using:
Efflux pumps
Porin size change
Enzyme point mutations to decrease binding affinity: for example, the gyrA gene in gonococcus
Usually class-wide
Pharmacokinetics/dynamics or FQs
Good bioavailability so useful orally
Mineral or cationic medications will block absorption (remember this problem with the tetracyclines?)
Moxifloxacin is excreted in the bile, which makes it a) not a good choice for urinary tract infections and b) useful in patients with renal failure
Black box warning of FQs
musculoskeletal toxicity most established in the medical literature is tendon rupture

