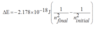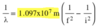Moles / Light Flashcards
1
Q
1 mole
A
- 6.022 x 1023 atoms
- As many particles as there are in 12.0 g of 12C
- Avagadro’s number
2
Q
Molar conversions
A

3
Q
A mole of a compound (covalent, ionic)
A
-
Covalent: Avogadro’s number of molecules
- 1 mole CO2 = 6.022 x 1023 CO2 molecules
- Ionic: Avogadro’s number of formula units
4
Q
How many moles of C, H, O in C6H12O2?
A
- C) 6 moles
- H) 12 moles
- O) 6 moles
5
Q
How to find molar mass
A
- Add for each element:
- Number of moles in the element * atomic mass (from periodic table, g/mole)
6
Q
Electromagnetic radiation
A
- Generated by moving electrons
- Can transport energy without a medium (unlike sound which requires matter to move through)
- Displays wave properties
- Diffraction
- Interference
7
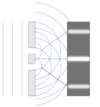
Q
Electromagnetic spectrum
A

8
Q
Features of waves
A
-
Wavelength: the distance between two identical points on two asjacent waves
- Symbol: lambda λ
- Unit: m, nm
- 109 nm in m

9
Q
Frequency
A
- The amount of crests that pass a point in one second
- Symbol: f, nu, ν
- Unit: s-1, Hz
10
Q
Relationship between wavelength and frequency
A
- All EM waves travel at speed of light
- c = 3.0 x 108 m/s
- c = λv
- Long wavelength = low frequency
- Short wavelength = high frequency

11
Q
Max Planck
A
- Solids emit radiation as heated
- Study relationships between wavelength and intensity of radiation emitted and temperature
- Energy is quantizes
- Lower frequency = intense light
12
Q
Quantization of energy
A
- Energy is released or absorbed in quanta
- Energy radiation is directly proportional to frequency (specific allowable energies)
- Quantized - values are restricted to certain quantities
13
Q
Energy equation (Planck’s constant)
A
- E = hv
- h = Planck’s constant = 6.626 x 10-34 J S
- v = frequency
14
Q
Wavelength vs energy vs frequency (red vs violet)
A
- RED: Large wavelength = low frequency = low energy
- VIOLET: Small wavelength = high frequency = high energy
15
Q
Photoelectric effect
A
- When light is shone on a metal, electrons are ejected from the metal
- The ejected electrons have a ceratin amount of kinetic energy
16
Q
Classical physics prediction
A
- Higher amplitude (brighter) = more energy
- Bright red = eject electrons, dim red = no electrons
- Bright blue = eject electrons, dim blue = no electrons
- Brighter light = higher energy of electrons popped off
17
Q
Blue vs red light actually observed
A
- Bright red = no electrons, dim red = no electrons
- Bright blue = eject electrons, dim blue = eject electrons (but fewer)
- Suggests that the ability of light to eject electrons depends on its frequency, not brightness
- Planck - energy and frequency directly related
- Einstein uses this to explain the photoelectric effect