Acids and Bases Flashcards
What does the pH scale measure?
(level 3 )
The acidity or alkalinity of a substance
What is the number range of the pH scale?
0-14

What is the pH range for acids?
Less than 7
(do NOT say 1-6)

What is the pH range for alkalis?
Greater than 7

(do NOT say 8-14)
What is a pH “indicator”?
A substance that changes colour in the presence of an acid or alkali.
What is the pH value for neutral solutions?
7
Name 3 indicators used in the lab
pH paper
universal indicator
red cabbage indicator
What does pH measure?
(level 4)
The concentration of H+ (aq) ions in a solution
What is the acidic ion?
The H+ (aq) ion
Definition of an acid
A solution with a higher concentration of hydrogen (H+) ions than hydroxide (OH-) ions

How is concentration of hydrogen ions represented?
By using square brackets
[H+ (aq)]
What effect does diluting an acid have on its pH value?
It increases it towards 7

What factor of dilution will increase the pH value of an acid by 1?
A dilution factor of 10
( adding 90ml of water to 10 ml of acid to make 100 ml for example)
What is the alkali ion?
The OH- (aq) ion
What makes solutions alkaline?
A solution with higher concentration of hydroxide ions than hydrogen ions

What effect does dilution of alkalis have on their pH value?
It decreases it towards 7

What factor of dilution will decrease the pH value of an alkali by 1?
A dilution factor of 10
( adding 90ml of water to 10 ml of alkali to make 100 ml for example)
Why do neutral solutions a have pH of 7?
Because they have equal concentrations of hydroxide ions and hydrogen ions
How is concentration of hydroxide ions represented?
By using square brackets
[OH- (aq)]
Why can the concentration of OH- (aq) NEVER be zero even with very low pH values?
Because
[H+ (aq)] x [OH- (aq)] = a number that is NOT zero
What is acid rain?
Rain that falls with a pH below 5.5
What are some uses of acids in food industry
- flavourings
- preservatives
Which 2 gases are mainly responsible for acid rain?
sulphur dioxide
nitrogen oxides

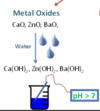
What is an “acidic oxide” ?
A NON-METAL oxide that is soluble in water and lowers the pH below 7.