Alcohol Flashcards
(38 cards)
What only type of alcohol react rapidly with halogen under normal condition (25 celcius)? What mechanism?
low weight, water soluble tertiary alcohol
SN1
What type of alcohol reacts smoothly with halogen to form haloalkene? Under what conditions and mechanism?
1º alcohol
heat reflux with water
SN2
Boiling point of alcohol in order (1º, 2º, 3º)
Why?
1º > 2º > 3º
the less hinder steric structure, the easier to have intermolecular H-bonding -> the higher boiling point
Solubility of alcohol (1 OH group, 2 OH group, 3 OH group) in water in order?
The more OH group, the more soluble in water
The ease of rxn of alcohol w/ halogen? (1º, 2º, 3º)
1º < 2º < 3º
What type of alcohol are not useful to make haloalkene? Why?
1º with ß branching and 2º
because they produce racemic products
What type of alcohol reacts with PBr3? Mechanism? Conditions?
1º 2º
SN2
at Oº C
Under what conditions does the water insoluble tertiary alcohol reacts with halogen? What mechanism?
at O° C and in either ether or THF solvents
SN1
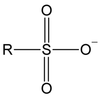
What solvents are used in rxn btw alcohol & SOCl2?
pyridine/ 3º amine
What type of alcohol reacts with SOCl2? Mechanism?
1º and 2º
SN2
Ease of acid catalyzed dehydration of alcohol in order (1º, 2º, 3º)?
1º < 2º < 3º
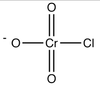
What compound is this? What is it character? What product it can help alcohol to make?
weak base, stable anion & good leaving group
haloalkene
What mechanisms for 2º & 3º alcohol react with halogen?
SN1
What type of alcohol react with H2CrO4 to give ketone?
What solvent?
2º alcohol
acetone
What type of alcohol react with PCC to give ketone?
What solvent?
2º alcohol
CH2Cl2
Why doesn’t 1º alcohol rxn with PCC result in carboxylic acid?
because PCC is used in solvent CH2Cl2 and not water
What mechanism does 2º, 3º alcohol undergo for acid catalyzed dehydration rxn? What is the major product?
E1 mechanism
the most substituent alkene (best stabilized carbocation intermediate)
What is the point of acid catalyzed dehydration of glycols? Mechanism?
to form ketone with the most stable carbocation intermediate
E1
What are the products of oxidation of 1º alcohol by H2CrO4?
aldehyde & carboxylic acid
What product does rxn of 2º alcohol with H2CrO4 give to?
ketone
What product does rxn of 3º alcohol with H2CrO4 give to?
none = no rxn
What compound is this? What type of alcohol does this compound reacts with? Mechanism? Conditions?
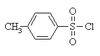
TsCl
1º, 2º
SN2
pyridine
What mechanism does 1º ß-branching undergo for acid catalyzed dehydration alcohol? For normal 1º alcohol?
E1
E1, E2
What type of alcohol and reagents react together to form aldehyde and final product carboxylic acid?
1º alcohol & H2CrO4 with acetone and water