C1 Oil, alkanes and fuels Flashcards
(30 cards)
What is crude oil?
Mixture of hydrocarbons
What is a mixture?
2 or more substances mixed but not chemically bonded together
What is the general formula for alkanes?
CnH2n+2
What is a hydrocarbon?
Compound made from only carbon and hydrogen
Are alkanes saturated or unsaturated?
Saturated
Draw a methane molecule.
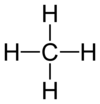
Draw an ethane molecule.

Give the formula for a propane molecule.
C3H8
Name C4H10.
Butane
What does the line in between C-C in a molecule show?
Covalent bond, a shared pair of electrons
What method is used to separate crude oil?
Fractional distillation
Describe how fractional distillation works?
Crude oil is heated to evaporate and then the different fractions are condensed because of their different boiling points
As the number of carbons increase in an alkane what happens to the boiling point?
It increases
As the number of carbons increase in an alkane what happens to the viscosity?
It increases, gets more sticky
As the number of carbons increase in an alkane what happens to the flammability?
Gets less flammable
As the temperature of oil increases what happens to the viscosity?
Gets less viscous, more runny
What are the products of complete combustion of hydrocarbons?
Carbon dioxide and water
What are the products of incomplete combustion of hydrocarbons?
Carbon monoxide and carbon (soot)
If crude oil contains some sulfur what combustion product will be made?
Sulfur dioxide
In combustion are carbon and hydrocarbon oxidised or reduced?
Oxidised as oxygen is added
High temperatures may cause oxides of nitrogen what type of pollution is caused by the oxides of nitrogen and sulfur dioxide?
Acid rain
What effect does this pollution have on the environment?
Weathering of rocks and buildings, and changes acidity of the soil
Carbon dioxide causes what type of pollution?
Global warming
Solid particles (soot or unburnt fuel) causes what type of pollution?
Global dimming


