Carcinogens, Cancer Epi, and Clinical Manifestations Flashcards
(139 cards)
What are the high-risk HPV types? Why?
- HPV 16, 18, 31
- Implicated in the pathogenesis of squamous cell carcinomas of the cervix, anogenital region, and head and neck (particularly tumors arising in the tonsillar mucosa)
- These cancers are sexually transmitted infections, caused by transmission of HPV
What % of women with breast cancer have no symptoms of it at time of dx and it is detected by screening with mammography?
55%
Describe how hepatocellular injury via chronic viral infection may lead to increased carcinogenesis.
- Leads to compensatory proliferation of hepatocytes
- Regenerative process aided by growth factors, cytokines, chemokines, and o/bioactive substances produced by activated immune cells -> promote cell survival, tissue remodeling, and angiogenesis
- Activated immune cells also produce other mediators, like ROS, that are genotoxic and mutagenic.
- 1 key molecular step is activation of NF-κB pathway in hepatocytes in response to mediators derived from the activated immune cells -> activation of this pathway in hepatocytes blocks apoptosis, allowing dividing cells to incur genotoxic stress and accumulate mutations
How has melanoma genome sequencing been used to reinforce the belief that sun exposure has an important causative role in this disease?

- Has revealed a very large number of mutations that appear to stem from non-templated, error-prone repair of pyrimidine dimers, reinforcing the belief that sun exposure has an important causative role in melanoma
What are nitrites?
- Used as food preservatives, and cause nitrosylation of amines contained in the food
- The nitrosoamines so formed are suspected to be carcinogenic
Once injected into a gastric mucosal epithelial cell, what does the virulence factor, CagA do?

- CagA PAI also encodes a type 4 secretion sys used to “inject” CagA into target cell upon H. pylori attachment
- CagA localizes to inner surface of cell membrane and undergoes tyrosine phosphorylation via Src family kinases -> phosphorylated CagA interacts with SHP-2 tyrosine phosphatase, so functionally active, triggering a host cell morphological change to more motile pheno
- This phenotype mimics effect produced by hepatocyte growth factor which may participate in various aspects of cancer, including metastasis (+ RAS, + MEK -> image)
- CagA a highly Ag protein associated with a prominent inflammatory response by eliciting IL-8 production
- Both CagA + and - strains; around 60% in Western countries positive, but majority in East Asian positive
How do translocations activate proto-oncogenes?
- While any type of chrom rearrangement can activate proto-oncogenes, translocation is most common mech
- Can activate proto-oncogenes in two ways:
1. Promoter/enhancer substitution: overexpression of proto-oncogene by swapping its regulatory elements with those of another gene, esp. one that is highly expressed
2. Formation of fusion gene: coding sequences of 2 genes fused in part or whole -> expression of novel chimeric protein w/oncogenic properties
What is going on in this stomach sample?

Cancer cells invading (top right and lower left)
What are the 3 acquired conditions that predispose to cancer?
- Chronic inflammatory disorders
- Precursor lesions
- Immunodeficiency states
How is epigenetics related to carcinogenesis?
- Epigenetics: factors other than the DNA seq that regulate gene expression -> important role in aspects of malignant phenotype, incl expression of cancer genes, control of differentiation and self-renewal, and even drug sensitivity and drug resistance
- Factors include:
1. Histone modifications catalyzed by enzymes associated with chromatin regulatory complexes
2. DNA methylation via DNA methyltransferases
3. Other proteins that regulate higher order org of DNA (e.g., looping enhancer elements on gene promoters)
How can proto-oncogenes and TSGs be tumor antigens?
- Products of altered proto-oncogenes, TSGs, and “passenger” genes translated in cyto of tumor cells and entering MHC I where they may be recognized via CD8
- May also enter MHC II in APCs that phagocytose dead tumor cells, and be recognized by CD4+ T cells, too
- Some pts have circulating CD4+ and CD8+ T cells that can respond to peptides from mutated oncoproteins like RAS, p53, and BCR-ABL.
How is HPV genome integration implicated in malignant transformation?
- While site of viral integration in host chromosomes is random, pattern of integration is clonal -> cells in which viral genome has integrated show significantly more genomic instability
- B/c integration site random, no consistent assoc with a host proto-oncogene, but, integration interrupts viral DNA in E1/E2 open reading frame, so loss of E2 viral repressor & overexpression of oncoproteins E6 and E7
- Oncogenic potential of HPV can largely be explained byactivities of the two viral genes encoding E6 and E7
What causes the carcinogenicity of UVB light?
- Due to formation of pyrimidine dimers in DNA
- If the energy in a photon of UV light is absorbed by DNA, a chemical rxn occurs, leading to covalent cross-linking of pyrimidine bases, esp. adjacent thymidine residues in the same strand of DNA
- Distorts DNA helix and prevents proper pairing of dimer with bases in opposite DNA strand
- Pyrimidine dimers are repaired via nucleotide excision repair pathway, but this pathway can be overwhelmed w/excessive sun exposure
Pause. And reflect. Also, memorize this graphic.

Good job!
What are these? How do they relate to neoplasia?

- Cafe au lait spots
- Neurofibromatosis Type 1 (NF1): auto dom disorder caused by mutations in TSG, neurofibromin, a negative regulator of the oncoprotein, Ras
- Disruption of neurofibromin function, leading to Ras hyperactivity; cardinal feature of NF1-associated tumors
- As would be anticipated for a tumor suppressor gene, sole normal neurofibromin allele mutated or silenced in tumors arising in NF1, which include neurofibromas
- Symptoms: learning disabilities, seizures, skeletal abnormalities, vascular abnormalities, pigmented iris nodules (Lisch nodules), and pigmented skin lesions (axillary freckling, café au lait spots) in various degrees
What is this? Describe the symptoms, mechanisms and associated neoplasias.

- Coalescing nests of intestinal carcinoid (carcinoid syndrome)
- Symptoms: attacks of cutaneous flushing (deep red erythema of face and neck; may become persistent erythema or cyanosis), diarrhea, cramps, nausea, cough, vomiting, etc.
- Mechanisms: serotonin, bradykinin (vasodilator)
- Neoplasias: bronchial adenoma (carcinoid), pancreatic, gastric carcinoma
What is the difference between lymphomas and leukemias?
- Lymphomas: solid hematologic malignancies
- Leukemias: liquid hematologic malignancies
How do HPV proteins promote many of the hallmarks of cancer?
- High-risk HPV types express oncogenic proteins that:
1. Inactivate tumor suppressors (p53, Rb, p21, p27)
2. Activate cyclins (E and A)
3. Inhibit apoptosis, and
4. Combat cellular senescence - NOTE: the primacy of HPV infection in the causation of cervical cancer is confirmed by the effectiveness of HPV vaccines in preventing cervical cancer
Diagnose this Pap.

LSIL (low-grade squamous epithelial lesion)
What is this?

Intestinal metaplasia due to H. pylori -> precursor to dysplasia and carcinoma
What is this? What is a potential precursor?
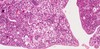
- Endometrial adenocarcinoma, potentially predicated by simple endometrial hyperplasia
What are some examples of the carcrinogenicity of radiation?
- Electromagnetic (x-rays, γ rays) and particulate (α particles, β particles, protons, neutrons) radiations
- Miners of radioactive elements in Rocky Mountains have 10x increased incidence of lung cancers
- Follow-up of survivors of atomic bombs: initially marked increase in incidence of certain leukemias after avg latent period of about 7 years; later, incidence of many solid tumors with longer latent periods (e.g., carcinomas of the breast, colon, thyroid, and lung) increased
I know this says what it is. Just observe.

Cervical squamous cell carcinoma
Describe the actions of the HPV E7 virulence factor.
- E7 protein effects complement those of E6, centered on moving cells past G1/S cell cycle checkpoint
- Binds **Rb **protein, displacing E2F transcription factors normally sequestered by Rb, promoting progression through cell cycle (high-risk HPV E7 has higher affinity for Rb than low-risk HPV)
- E7 also inactivates CDK inhibitors p21 and p27
- High-risk HPVs (types 16, 18, and 31) E7 also binds and presumably activate cyclins E and A








































