cardiology pathology Flashcards
(48 cards)
congenital heart diseases
what are the 5 Ts?
Early cyanosis, blue babies become evident immediately after birth
need urgent surgical tx and/or maintenance of PDA
5 Ts
- Truncus arteriosus
- transposition
- tricupid atresia
- tetralogy of Fallot
- TAPVR-Total anomalous pulmonary venous return (TAPVR) is a birth defect of the heart. In a baby with TAPVR, oxygen-rich blood does not return from the lungs to the left atrium. Instead, the oxygen-rich blood returns to the right side of the heart.
congenital heart disease:
failure of aorticopulmonary septum formation. most patients have accompanying VSD
- heart that does not have pulmonary trunk and aorta
R-L shunt
persistent truncus arteriosus
aorta leaves RV (anterior) and pulmonary trunk leaves LV (posterior)–> separation of systemic and pulmonary circulation
this condition is not compatible with life unless there is a shunt to allow mixing of blood (VSD, PDA, patent foramen ovale)
due to failure of aorticopulmonary septum to spiral
R-L shunt
D-transposition of great vessels
absence of tricuspid valve and hypoplastic RV
pt needs ASD and VSD for viability
R-L shunt
tricuspid atresia
anterosuperior displacement of infundibular septum and most common cause of childhood cyanosis
- pulmonary infundibular stenosis (most important determinant for prognosis)
- Right ventricular hypertrophy (boot-shaped heart on CXR)
- overriding aorta- aorta is positioned directly over a ventricular septal defect (VSD), instead of over the left ventricle.
- VSD
pulmonary stenosis forces R-L shunt across VSD–> RVH (often caused by crying, fever, exercise due to exacerbation of RV outflow obstruction)
squatting increases afterload, decrease shunting, improves cyanosis
associated with DiGeorge and need early surgical correction
DiGeorge syndrome- 22q11.2 deletion
R-L shunt
Tetralogy of Fallot
congenital heart disease
pulmonary veins drain into right heart circulation (SVC, coronary sinus, etc) associated with ASD and sometimes PDA to allow R-L shunting to maintain CO
R-L shunt
total anomalous pulmonary venous return
congenital heart disease
characterized by displacement of tricuspid valve leaflet downward into RV, “atrializing” the ventricle
associated with TR, accessory condution pathway, right HF
caused by lithium exposure in utero
R-L shunt
Ebstein anomaly
Left-Right shunts: acyanotic at presentation; cyanosis may occur years later
R-L: early cyanosis
L-R: later cyanosis
most common congenital cardiac defect
asymptomatic at birth and manifest weeks or later or remain asymptomatic throughout life
most are self-resolving; larger lesion may lead to LV overload and HF
L-R shunt
ventricular septal defect
O2 saturation increase in RV and pulmonary artery.
frequency: VSD> ASD> PDA
congenital heart disease
defect in interatrial septum
-wide, fixed split S2
Ostium secundum defects most common
O2 saturation increased in RA, RV and pulmonary artery. may lead to paradoxical emboli
associated with Down syndrome
distinct from foramen ovale in that septa are missing tissue rather than unfused
L-R shunt
atrial septal defect
PDA
fetal period, shunt is right to left (normal)
in neonatal period, decreased pulmonary vascular ressitance–> shunt becomes left to right–> progressive RVH and/or LVH and HF
associated with continuous, machine-like murmur
patency is maintained by PGE synthesis and low O2 tension.
uncorrected PDA can eventually result in late cyanosis in the lower extremities (differential cyanosis)
Endomethacin (indomethacin) ends patency,
PGE keeps it open- could be needed to sustain life such as in transposition of the great vessels
uncorrected Left-Right shunt (VSD, ASD, PDA) –> increase pulmonary blood flow–> pathologic remodeling of vasculature–> pulmonary arterial hypertension.
RVH occurs compensate–> shunt becomes right to left shunt
cause late cyanosis, clubbing and polycythemia
Eisenmenger syndrome
aortic narrowing near insertion of ductus arteriosus (juxtaductal) associated with bicuspid aortic valve and turner syndrome.
HTN of upper extermities and weak, delayed pulse in lower extremities (brachial-femoral delay)
with age, the intercostal arteries enlarge due to collateral circulation. arteries erode ribs--> notched appearance on CXR.
complication include HF, increase cerebral hemorrhage (berry aneurysm), aortic rupture and possible endocarditis
coarctation of aorta
- alcohol exposure in utero (fetal alcohol syndrome)
- congenital rubella
- down syndrome
- infant of diabetic mother
- marfan syndrome
- prenatal lithium exposure
- Turner syndrome
- William syndrome
- 22q11 syndrome
- VSD, PDA, ASD, teterlogy of Fallot
- PDA, pulmonary artery stenosis, septal defects
- AV septal defect (endocardial cushion defect), VSD, ASD
- Transposition of great vessel, VSD
- MVP, thoracic aotic aneurysm and dissection, aortic regurgitation
- Ebstein anomaly
- biscupsid aortic valve, coarctation of aorta
- supravalvular aortic stenosis
- Truncus arteriosus, tetralogy of Fallot
HTN
persistent systolic BP > 130 and or diastolic > 80
RF: advancing age, diabetes, inactivity, excess salt, excess alcohol, cigarette, FH, African> causcasian> Asian
90% of HTN are essential- related to increase CO and TPR
remaining are seconday- renal/renovascular diseases like fibromuscular dysplasia (string of beads appearance of renal arteries in women of child-bearing age) and atherosclerotic renal artery stenosis or primary hyperaldoseronism
Hypertensive urgency- severe (>180/>120) HTN without acute end-organ damage
Hypertensive emergency- severe HTN with evidence of acute-end organ damage (encephalopathy, stroke, retinal hemorrhages and exudates, papilledema, MI, HF, aortic dissection, kidey injury, microangiopathic hemolytic anemia, eclampsia)
hyperlipidemia signs
xanthomas-plaques composed of lipid-laden histiocytes in skin and especially the eyelids (xanthelasma)
Tendinous xanthoma-lipid deposit in tendon esp, achilles
corneal arcus-lipid deposit in corea. common in elderly (arcus senilis) but earlier in life with hypercholesterolemia

arteriosclerosis-hardening of arteries, with arterial wall thickening and loss of elasticity
arteriolosclerosis-commonly affects small arteries and arterioles.
2 types of arteriolosclerosis:
1. hyaline (thickening of vessel walls in essential HTN or diabetes mellitus) and
- hyperplastic (onion skinning in severe HTN with proliferation of smooth muscle cells)
Monckeberg sclerosis (medial calcific sclerosis)- uncommon. affects medium arteries, calcification of the internal elastic lamina and media of arteries–> vascular stiffening without obstruction.
pepestern appearance on xray and does not obstruct blood flow. intima is not involved

Atherosclerosis
disease of elastic arteries and large-medium sized muscular arteries; a form of arterosclerosis caused by buildup of cholesterol plaques
abdominal aorta> coronary artery> popliteal artery> carotid artery
sx: angina, claudication-(Pain, commonly in the legs, caused by too little blood flow, usually during exercise. Often indicates peripheral artery disease.), but can be asymptomatic
progression: endothelial cell dysfunction–> marcophage and LDL accumulation–> foam cell formation–> fatty streak–> smooth muscle cell migration (involves acculumation PDGF and FGF), proliferation, and extracellular matrix deposition–> fibrous plaque–> complex atheroma
complication: aneurysm, ischemia, infarcts, peripheral vascular disease, thrombus, emboli
aortic aneurysm
localized dilation of the aorta causing abdominal and or back pain, which is a sign of leaking, dissection or imminent rupture
abdominal aortic aneurysm- associated with atherosclerosis. palpable pulsatile abdominal mass
most are infrarenal (distal to origin of renal arteries)
thoracic aortic aneurysm-associated with c_ystic medial degeneration_. risk factor include HTN, Bicuspid aortic valve, connective tissue disease (marfan syndrome)
also associated with 3’ syphilis (obliterative endarteritis of vasa vasorum) aortic root dilation may lead to aortic valve regurgitation
Traumatic aortic rupture-due to trauma and or deacceleration injury, most commonly at aortic isthmus (proximal descending aorta just distal to origin of left subclavian artery)
xray may should widened mediastinum.
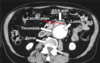
longitudinal intimal tear forming a false lumen associated with HTN, bicuspid, Marfans,
can present with tearing, sudden onset of chest pain that radiates to the back .
CXR shows mediastinal widening.
stanford type A: (proximal)- involves ascending aorta. may extend to aortic arch or desencing aorta. may result in acute aortic regurg or cardiac tamponade
type B: (distal)- involves only descending aorta (below left subclavian artery) treated medically with beta-blockers, then vasodilators
ischemic heart disease manifestation
angina- chest pain due to ischemic myocardium secondary to coronary artery narrowing or spasm; no myocyte necrosis
1. stable: usually secondary atherosclerosis (>70% occulsion)- exertional chest pain in classic distribution (usually with ST depression on EKG) resolving with rest or nitroglycerin
2. vasospastic (also known as prinzmetal or variant)- occurs at rest secondary to coronary artery spasm; transient ST elevation on EKG, smoking is risk factor; HTN and hypercholesterolemia are not. trigger include cocaine, alcohol, triptans. treat with Ca2+ channel blockers, nitrates and smoking cessation
unstable: thrombosis with incomplete coronary artery occlusion, +/- ST depression and T wave inversion on EKG but no cardiac biomarker elevation (unlike NSTEMI); infrequency or intensity of chest pain or any chest pain at rest
coronary steal syndrome- distal to coronary stenosis, vessels are maximally dilated at baseline. adminsteration of of vasodilators (dipyramidole, regadenoson) dilates normal vessels–> blood is shunted toward well-perfused areas--> ischemia myocardium perfused by stenosed vessels.
principle behind pharmacologic stress tests with coronary vasodilators
ischemic heart disease manifestations
sudden cardiac death- death from cardiac within 1 hour onset on sx. most commonly due to lethal arrhythmia (VF). associated with CAD, cardiomyopathy (hypertrophic, dilated) and hereditary ion channelopathies (long QT syndrome, Brugada syndrome). prevent with ICD
Chronic ischemic heart disease- progressive onset of HF over many years due to chronic ischemic myocardial damage
myocardial infarction- most often rupture of coronary artery atherosclerotic plaque--> acute thrombosis. increase cardiac biomarkers (CK-MB, troponins) are diagnostic.
non-STEMI-subendocardial infarcts- subendocardium (inner 1/3) especially vulnerable to ischemia. ST depression on EKG
ST- segment elevation MI (STEMI)- transmural infarcts, full thickness of myocardial wall involved. ST elevation, Q waves on EKG
evolution of myocardial infarction
occluded LAD>RCA>LCX
sx: diaphoresis, nausea, vomiting, severe retrosternal pain, pain in left arm and or jaw, SOB, fatigue
0-24hr- Gross: none. microscopically: early coagulative necrosis, wavy fibers, reprofusion injury, complication: Venticular arrhythmia, HF, cardiogenic shock
Pathoma: <4 hours: gross changes: none, microscopic: none. complication: cardiogenic shock (massive infarction) congestive heart failure, arrhythmia
4-24 hours: gross changes: dark discoloration, microscopic: coagulative necrosis. complication arrhythmia
pathoma: 1-3 days: gross changes: yellow pallor, microscopic changes: neutrophils, complication: fibrinous pericarditis represented as chest pain with friction rub
pathoma: 4-7 days: gross: yellow pallor, microscopic: marcophages, complication: rupture of ventricular free wall (lead to cardiac tamponade), interventicular septum (leads to shunt) or papillary muscle (leads to mitral insufficiency)
1-3 weeks gross: red border emerges as granulation tissue enters from edge of infarct, microscopic: granulation tissue
months: gross changes: white scar, microscopic changes: fibrosis, complication: Aneurysm, mural thrombus or dressler syndrome
in first 6 hours: EKG is gold standard
cardiac troponin I rise after 4 hours (peaks 24hrs) last form 7-10 days
CK-MB- rise 6-12 hours (peak 16-24 hr) good for diagnosing reinfarction following MI because level return to normal in 48 hours
EKG localization of STEMI
location of infacrion and where to see ST elevation or Q waves:
anteroseptal (LAD): V1-2
anteroapical (distal LAD): V3-4
anterolateral (LAD or LCX): V5-6
Lateral (LCX): I, aVL
inferior (RCA) II, III, aVF
posterior (PDA): V7-9, ST depression in V1-3 with tall R waves

myocardial infarction complication
cardiac arrhythmia- within first few days after MI, important cause of death before reaching the hospital and within 24 hours post-MI
Post-infarction fibrinous pericarditis: 1-3 days friction rub
papillary muscle rutpure: 2-7 days: posteriomedial papillary muscle rupture. increase risk due to single blood supply from posterior descending artery. can result in severe mitral regurg
interventricular septal rupture: 3-5 days: macrophage-mediated degradation–> VSD–> increase O2 sat and pressure in RV
ventricular pseudoaneurysm formation: 3-14 days: free wall rupture contained by adherent pericardium or scar tissue: decrease CO, increase risk of arrhythmia, embolus from mural thrombus
ventricular free wall rupture: 5-14 days: free wall rupture–> cardiac tamponade. LV hypertrophy and previous MI protect against free wall rupture. Acute form usually leads to sudden death
true ventricular aneurysm: 2wks to several months: outward bulge with contraction (dyskinesia) associated with fibrosis
Dressler syndrome: several weeks: autoimmune phenomenon resulting in fibrinous pericarditis
LV failure and pulmonary edema: can occur secondary to LV infarction, VSD, free wall rupture, papillary muscle rupture with mitral regurg.
unstable angina/NSTEMI- anticoagulation (heparin), antiplatelet therapy (aspirin) + ADP (adenosine diphosphate) receptor inhibitor (clopidogrel), B-blocker, ACE inhibitors, statins. symptom control with nitroglycerin and morphine
STEMI- in addition to above, reperfusion therapy most important (percutaneous coronary intervention preferred over fibrinolysis)
Most common cardiomyopathy (90% of cases). Often idiopathic or familial. Other etiologies include chronic Alcohol abuse, wet Beriberi, Coxsackie B viral myocarditis, chronic Cocaine use, Chagas disease, Doxorubicin toxicity (ABCCCD), hemochromatosis, sarcoidosis, thyrotoxicosis, peripartum cardiomyopathy.
Findings: HF, S3, systolic regurgitant murmur, dilated heart on echocardiogram, balloon appearance of heart on CXR.
Leads to systolic dysfunction. Dilated cardiomyopathy displays eccentric hypertrophy (sarcomeres added in series). ABCCCD.
Takotsubo cardiomyopathy: broken heart syndrome—ventricular apical ballooning likely due to increased sympathetic stimulation (eg, stressful situations).
Treatment: Na+ restriction, ACE inhibitors, β-blockers, diuretics, digoxin, ICD, heart transplant.
dilated cardiomyopathies
60–70% of cases are familial, autosomal dominant (most commonly due to mutations in genes encoding sarcomeric proteins, such as myosin binding protein C and β-myosin heavy chain).
Causes syncope during exercise and may lead to sudden death (eg, in young athletes) due to ventricular arrhythmia.
Findings: S4, systolic murmur. May see mitral regurgitation due to impaired mitral valve closure.
Diastolic dysfunction ensues. Marked ventricular concentric hypertrophy (sarcomeres added in parallel), often septal predominance. Myofibrillar disarray and fibrosis.
Physiology of HOCM—asymmetric septal hypertrophy and systolic anterior motion of mitral valve –> outflow obstruction –> dyspnea, possible syncope.
Other causes of concentric LV hypertrophy: chronic HTN, Friedreich ataxia.
Treatment: cessation of high-intensity athletics, use of β-blocker or non-dihydropyridine Ca2+ channel blockers (eg, verapamil). ICD if patient is high risk
hypertrophic obstructive cardiomyopathy
Postradiation fibrosis, Löffler endocarditis-(abnormal endomyocardial infiltration of eosinophils, with subsequent tissue damage from degranulation, eventually leading to fibrosis,) Endocardial fibroelastosis (thick fibroelastic tissue in endocardium of young children), Amyloidosis, Sarcoidosis, Hemochromatosis (although dilated cardiomyopathy is more common)
(Puppy LEASH).
Diastolic dysfunction ensues. Can have low-voltage ECG despite thick myocardium (especially in amyloidosis).
Löffler endocarditis—associated with hypereosinophilic syndrome; histology shows eosinophilic infiltrates in myocardium.
restrictive/infiltrative cardiomyopathy


