Ch1 Flashcards
(122 cards)
What are the six distinguishing features of living organisms?
A high degree of chemical complexity and microscopic organization.
Systems for extracting, transforming, and using energy from the environment.
Defined functions for each of an organism’s components and regulated interactions among them.
Mechanisms for sensing and responding to alterations in their surroundings.
A capacity for precise self-replication and self-assembly.
A capacity to change over time by gradual evolution.
What boundry does the plasma membrane define?
The perhiphery of the cell, separating the contents from the surroundings.
What are the universal features of living cells?
A nucleous or nucleoid, a plasma membrane, cytoplasm.

The cytosol is defined as what portion of the cytoplasm?
The cytosol is defined as that portion of the cytoplasm that remains in the supernatant after gentle breakage of the plasma membrane and centrifugation of the resulting extract at 150,000 g for 1 hour.

What remains in the supernatant of cytoplasm centrifuged at 150,000 g for one hour?
The cytosol, the supernatant of cytoplasm, a concentrated solution of enzymes, RNA, monomeric subunits, metabolites, and inorganic ions.

What forms the pellet of cytosol when centrifuged at 150,000 g for one hour?
After the cytosol (the supernatant) is removed, particles and organelles are what remains:
Ribosomes, storage granules, mitochondria, chloroplasts, lysosomes, endoplasmic reticulum.

Define the two types of phototrophs by carbon source and give examples of each:
Autotrophs: carbon from CO2 (inorganic).
ex: cyanobacteria, vascular plants
Heterotrophs: carbon from organic compounds.
ex: purple bacteria, green bacteria

Define the two types of chemotrophs by energy source and give examples of each:
Lithotrophs: oxidise inorganic fuels.
ex: sulfur bacteria, hydrogen bacteria
Organotrophs: oxidise organic fuels.
ex: most bacteria, all nonphototrophic eukaryotes
Both types may be either autotropic or hetertrophic with regard to carbon source used for catabolism.

List common features of bacterial cells:
Nucleoid, ribosomes, pili, flagella, cell envelope (Gram - or Gram +)

ribosome
ribosomes synthesise protein from an RNA message, 70S in bacteria, 80S in eukaryotes.

nucleoid
the nucleoid contains a single, simple, long circular DNA molecule, not membrane bound

pili
pili provide points of adhesion to surface of other cells.

flagella
flagellum are used propel cell through its surroundings.

Gram + vs Gram -
Gram - : Inner membrane, thinner (relative to Gram +) peptidoglycan layer, LPS (lippopolysaccharide) outer membrane.
Gram + : Inner membrane, thinner (relative to Gram -) peptidoglycan layer, no outer membrane.

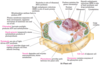
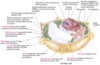
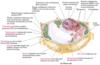
peroxisome
peroxisome is a vesicle present in cytoplasm that oxidises fatty acids

cytoskeleton
cytoskeleton supports the cell, aids in movement of organelles

nucelar envelope
nucelar envelope segregates chromatin (DNA protein) from cytoplasm

lysosome
lysosome is a vesicle present in cytoplasm that degrades intracellular debris (animal cells only)

Golgi complex
Golgi complex processes, packages, and targets proteins to other organelles or for export

smooth endoplasmic reticulum
smooth endoplasmic reticulum is the site of lipid synthesis and drug metabolism

rough endoplasmic reticulum
rough endoplasmic reticulum is the site of much protein synthesis

mitochondrion
mitochondrion oxidises fuels to produce ATP

transport vesicle
transport vesicle shuttles lipids and proteins between the endoplasmic recticulum, Golgi complex, and plasma membrane

nucleolus
nucleolus is the site of ribosomal RNA (rRNA) synthesis