Chapter 1: Amino Acids, Peptides, and Proteins Flashcards
(54 cards)
What are molecules that contain these two functional groups: an amino group (-NH2) and a carboxyl group (-COOH)?
Amino acids.
In a-amino acids, the amino group and carboxyl group are bonded to which carbon
The a-carbon of the carboxylic acid.
The a-carbon has two other groups attached to it. What are they?
A hydrogen atom and a side chain (-R group) which is specific to each amino acid.
Side chains determine what about amino acids?
They determine the properties of amino acids, and therefore their functions.
The 20 a-amino acids that are encoded by the human genetic code are also called what?
Proteinogenic amino acids.
For most amino acids, the a-carbon is a what center, as it has four different groups attached to it?
Chiral (stereogenic) center.
Because most amino acids have a chiral a-carbon, most amino acids are what?
Optically active.
What amino acid has an achiral a-carbon atom due to hydrogen being its R group?
Glycine
The stereochemistry for all chiral amino acids used in eukaryotes is?
L-amino acid, thus the amino group is draawn on the left in a Fischer projection.
In the Cahn-Ingold-Prelog system, L-amino acids translates to which absolute configuration?
(S) absolute configuration.
What L-amino acid has an (R) absolute configuration due to the -CH2SH group having higher priority over the -COOH group?
Cysteine.
What are the 7 proteinogenic amino acids that have nonpolar, nonaromatic side chains?
Glycine, alanine, valine, leucine, isoleucine, methionine, proline.
Glycine has what as it’s side chain?
Hydrogen atom in its side chain, thus making it achiral.
Which amino acids have alkyl side chains containing one to four carbons?
Valine, alanine, leucine, isoleucine.
Why is methionine relatively nonpolar?
Methionine is one of two amino acids with a sulfur atom in its chain and because a methyl group is attached to the sulfur, it’s relatively nonpolar.
Why is proline special?
Proline forms a cyclic amino acid. The amino nitrogen becomes a part of the side chain, forming a five-membered ring. The ring places constraints on the flexibility of proline, which limits where it can appear in a protein and can have significant effects on proline’s role in secondary structure.
What amino acid is pictured here?

Alanine.
What amino acid is pictured here?

Glycine.
What amino acid is pictured here?

Valine.
What amino acid is pictured here?

Leucine.
What amino acid is pictured here?
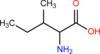
Isoleucine.
What amino acid is pictured here?

Methionine.
What amino acid is pictured here?

Proline.
What amino acid is pictured here?

Tryptophan.














