Chemical reactivity and mechanisms Flashcards
(61 cards)
Heterolytic bond cleavage
Results in forming ions & use only a single head curved arrow

Bond dissociation energy
The energy required to break a covalent bond through hommolytic bond cleavage
Homolytic Bond cleavage
Results in forming two uncharged radicals (Use two half curved arrows)

Enthaply (ΔH)
Heat at constant pressure
The ΔH0 is known as the ______________
Standard bond dissociation (under standard conditions like 1 atm)
Heat of reaction
The total change in enthaply for a reaction
Entropy
Measure of disorder associated with a system
Spontaneous reaction
Increases total entropy (ΔStot = Ssys + Ssurr) (ΔStotal has to be positive for a reaction to be spontaneous)
Between liquids, solids, & gases list it in increasing entropy
Ssolid < Sliquid gas (from least to greatest)
Under constant pressure what is the equation used for ΔSsurr ?
ΔSsurr = -ΔHsys / T
What is the equation for Gibbs free energy?
ΔG = ΔH - TΔS (ΔG must be negative for a reaction to be spontaneous)
Exergonic
A reaction where ΔG is negative
Endergonic
A reaction where ΔG is positive
Equilibrium constant (Keq)
Keq = [Products] /[Reactants]
Equation relating equilibrium constant to ΔG
ΔG = -RTlnKeq
With the equation relating ΔG & Keq if ΔG is negative then the __________ are favored (Keq > 1) & if ΔG is positive the _________ will be favored (Keq < 1)
Products, Reactnats
What is the rate equation?
R = k[Reactants] (R = k[A] *[B]*)
What are the factors that affect the rate constant?
- Energy Activation (Ea)
- Temperature
- Steric Considerations
How does Ea (activation energy) affect the rate of a reaction?
The activation energy is the minium energy required for a reaction to occur so the lower the Ea the faster the reaction & the larger the Ea the slower the reaction
How does the temperature affect the rate of a reaction?
The rate constant (k) is directly proportional to temp. & the rate of a reaction so increase the temp will increase the rate constant & speed up the rate of the reaction & vis versa when decreased
Catalysts
Speeds up the reaction without being consumed (Enzymes are a type of calalysts)
Transition state on a energy diagram
Highest peaks on the graph
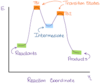
Intermediate on a energy diagram
Peaks where it concaves up on the graph

The transition state is where the ____________ occurs where bonds are broken & formed
Reaction







