Chemistry Flashcards
(48 cards)
What’s the formula for Dalton’s law
Pgas = XgasPtotal ; Xgas = n gas/ n total
gases conversion for 1 atm –> mmHg –>
760 mmHG; 10^5 Pa
A climber using bottled oxygen accidentally drops the oxygen bottle from an altitude of 4500 m. If the bottle fell straight down this entire distance, what is the velocity of the 3 kg bottle just prior to impact at sea level?
use 1/2mv^2 = mgh to solve
why does volume and densities of gas is not constant like fluid and solids?
because gas is compressible
Warm air holds (more/less) H2O than cold air?
more
Define positive control
a control group that is not exposed to the experimental etreatment but t
what is this functional group?

Amine
What is this functional group?

Amide
What is this functional group?
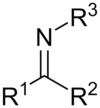
Imine
What is this functional group?

Thioether
What is this functional group?

Ester
What is this functional group?

Anhydride
When 2 moles of HF are added to 100 mL of water, the resulting solution has a pH equal to 4. What is the percent dissociation of HF?
KNOW that percent dissociation is [H+]/[HF] x 100%.
You need to find the molarity of HF then the [H+] is 10^-4 M
Equation for power : If the bicep exercises last 5 minutes, what is the total work performed by the patient’s muscle?
P = W / T
Equation for work energy theorem: An object with a mass of 10 kg is rolled down a frictionless ramp from a height of 3 meters. If a fasctory worker at the bottom of the ramp slows the object until it stops. How much work must the factory worker have done?
Wnet = KEnet ; or Wnet = mgh in this case because KE is from PE.
Formula for wavelength of a photon
- ALWAYS convert eV energy to J before calculating
- E=hc/wavelegnth
- 1 eV to J will be given
Equation for half lives
- How much of a sample is lost ?
- How much of a sample remain?
n = number of half life
Sample lost = 1- 1/2^n
Sample remain = 1/2^n
What is radioactive decay?
The spontaneous transformation from one element to another by changing the protons
IRREVERSIBLE
What is Alpha decay ?

What does Gamma decay look like ?
What is the formula for beta decay ?

What is beta minus?
A neutron is converted into a proton and a beta particle ( electron) is emitted = charge preserved

What is electron capture ?
The nucleus grabs an electron which changes a proton into a neutron
what type of group ( Electron withdrawing/donating) will increase the ka of a functional group like carboxylic acid?
Electron withdrawing groups ( F, Cl, S, N, S=O, NO2R ,etc)






