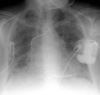
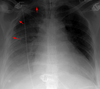
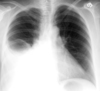
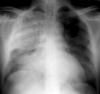
✅☢ CRX Flashcards
(66 cards)
- Firsts, 3. Quality, 4. Airway, 5. Bone, 6. CadioMediastinum 7. Diaohragm, 8. Effusions (Pleural, pericardial), 9. Fields (lung), 10. Pneumonia, 13. Groung glass, SPN, TB, PH, PE, Pneumo, IPF, emohysema, Mediastinal mass, pneumopericardium, dia hernia, hilar adenopathy, LC, abscess, tubes lines drains, pleural effusion, pericardial effusion, pulmonary edema, cadiogenic edema, CHF, cephalization, kerley b, interstitial edema, air bronchogram, alveolar edema, ARDS, atelectasis, 64., 67. aspiration syndromes
Index
Firsts
Turn off stray lights, optimize room lighting, view images in order
Patient data:
Name
Date
PA/AP
Uprigt/supine
Quality
Rotated? Symmetrical clavicles / clavicular heads
Penetration? Thoracic spine seen through heart
All areas included? (costophrenic angles)
Inflation / Inspiratory effort (3 cm diaphragmatic curvature; 8 (9) -10 posterior ribs in nl)
Airway
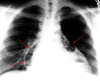
Midline / Deviation of the trachea (related to a mediastinal hematoma)
Masses in the airway
Airway compression
Bone
Lesions or fractures
Clavicles
Scoliosis?
Soft tissue calcification
RUG (gallstones, free air)
CardioMediastinum
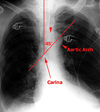
Borders (R, aortic knob, pulmonary a, l. atrium, l ventricle)
Lines (R paratracheal, Azygous, SVC, Aorta, Azygoesophageal line, descending aortic line)
Cardiomegaly (Cardiothoracic ratio)
RA, LV, LA
Mediastinal contour: width? mass?
Widened mediastinum:
Loss of the normal clear aortic arch contour “knob”
Loss of the appearance of a normal descending thoracic aorta (no ‘lateral aortic silhouette’ is seen).
Deviation of nasogastric tubes to the right (Indicating a mediastinal hematoma pushing the esophagus to the right side).
Left apical pleural ‘capping’
There is is a rind of fluid above the left lung apex where blood has tracked posteriorly over the left apex.
Signs of a supine pleural effusion
Diaphragm
Sharp border
Costophrenic angles sharp bilaterally
Air under diaphragm
Effisions (Pleura, Pericardial)
Pleural, pericardial
Lucencies (pneumothorax)
Thickeing, nodularity, calcification, or effusions
Fields (Lung)
Lung zones symmetrical?
Parenchyma (focal or diffuse abnormalitis)
Interstitial and vascular markings (size, prominence)
Lucency (pneumothorax), cavity, or abnormal shadowing (companion shadow of the second rib)
Hila (l higer than right; branching pattern)
Infiltrates
Alveolar process (fluffy)[filled alveoli]
Reticular process (lacy)[cell]
Pneumonia
Pneumonia is a space occupying lesion without volume loss. Pneumonia is caused by bacteria, viruses, mycoplasmae and fungi.
Ddx: Airspace filling not distinguishable radiographically: fluid (inflammatory), cells (cancer), protein (alveolar proteinosis) and blood (pulmonary hemorrhage).
The x-ray findings of pneumonia are airspace opacity, lobar consolidation, or interstitial opacities. There is usually considerable overlap.
Lobar - classically Pneumococcal pneumonia, entire lobe consolidated and air bronchograms common
An “Air bronchogram” is a tubular outline of an airway made visible by filling of the surrounding alveoli by fluid or inflammatory exudates. Six causes of air bronchograms are: Lung consolidation (PNA), pulmonary edema, nonobstructive pulmonary atelectasis, severe interstitial disease, neoplasm, and normal expiration.
Lobular - often Staphlococcus, multifocal, patchy, sometimes without air bronchograms
Interstitial - Viral or Mycoplasma; latter starts perihilar and can become confluent and/or patchy as disease progresses, no air bronchograms
“Ground Glass” is a radiology descriptive term (used in both chest radiographs and CT imaging) to indicate that blood vessels are not obscured as would be the case in alveolar lung opacities. Both atypical bacterial and viral organisms may produce pneumonias that differ radiographically from more common bacteria such as pneumococcus. They may produce a ground glass appearance and increased interstitial markings. The CXR appearance of Pneumocystis pneumonia is typically bilateral, diffuse interstitial (“reticular”) or ground glass opacities.
Aspiration pneumonia - follows gravitational flow of aspirated contents; impaired consciousness, post anesthesia, common in alcoholics, debilitated, demented pts; anaerobic (Bacteroides and Fusobacterium)
Diffuse pulmonary infections - community acquired (Mycoplasma, resolves spontaneoulsy) nosocomial (Pseudomonas, debilitated, mechanical vent pts, high mortality rate, patchy opacities, cavitation, ill-defined nodular) immunocompromised host(bacterial, fungal, PCP)
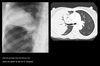
Pneumonia in the ICU
Nosocomial pneumonias by definition occur 3 days after admission. They differ from community-acquired pneumonias in both etiology and prognosis. Patients in the ICU are often relatively immunocompromised secondary to their primary disease and are subject to iatrogenic factors which increase their sucseptabilty to pneumonia-causing pathogens. These include the following: endotracheal tubes, which defeat many patient defense mechanisms; medications used to reduce gastric acid, which may promote bacterial growth in the stomach; and the use of antibiotics, which may selectively encourage the growth of some pathogenic bacteria. Nosocomial pneumonia presents a great concern for the intensivist and is the leading cause of infectious death in hospitals. Unlike community-acquired pneumonias, which usually are caused by gram-positive species, nosocomial pneumonias are often polymicrobial and caused by gram-negative enteric pathogens. The offending organisms often include Pseudomonas species, E-coli, Klebsiella species, and Proteus species. Traditional clinical indicators of pneumonia, including fever, elevated white blood cell count, and positive sputum cultures are often masked by severe underlying disease. The chest film must be correlated with clinical data in order to make the diagnosis of pneumonia in the ICU patient.
In a supine patient who has aspirated, the common locations of pneumonia are the psoterior segment of the upper lobe and superior segment of the lower lobe
Radiographic Appearance of Pneumonia
The radiographic appearance of pneumonia may be difficult to differentiate from atelectasis or early ARDS. Classically, pneumonia first appears as patchy opacifications or ill-defined nodules. It is often multifocal and bilateral, occurring most often in the gravity dependent areas of the lung. This feature makes it difficult to distinguish from atelectasis or apulmonary edem. E-coli and pseudomonas species can rapidly involve the entire lung. Their symmetric pattern often simulates pulmonary edema. The presence of patchy air space opacities, air bronchograms, ill-defined segmental consolidation or associated pleural effusion support the diagnosis of pneumonia. Occassionally, in gram-negative pneumonias small luciencies may be found within consolidated lung which may represent unaffected acini or areas of air trapping. This is particularly likely to occur in patients with underlying COPD. However, these must be distinguished from lucencies created by cavitation and abscess formation.
Complications of nosocomial pneumonias can have severe consequences and require immediate attention. Unlike community acquired pneumonia, pleural effusions caused by gram-negative organisms are more likely to represent empyema and therefore require drainage. Other complications of nosocomial pneumonias include lung abscess formation and bronchopleural fistulas.

Solitary Pulmonary Nodule
A differential of possible etiologies is as follows:
Granuloma – usually caused by fungal infections like histoplasmosis or tuberculosis
Lung Carcinoma
Solitary metastasis – usually from colon, breast, kidney, ovary, or testis
Round pneumonia
Abscess
Round atelectasis
Hamartoma – popcorn calcification is sometimes seen
Sequestration
Arteriovenous malformation
Other things can cause an apparent nodule but are actually outside the lung including:
Fluid in an interlobar fissure
Pleural plaques – small, often calcified, plate-like surfaces on the pleura often caused by asbestos fibers that invade the pleura from the lungs
Skin lesions – nipple shadow, mole, lipoma, etc.
Low Risk Patient
≤ 4mmNo follow-up needed
4-6mm12 mo; if no change - stop
6-8mm6-12 mo; no change - follow-up at 18-24 mo
> 8mmCT follow-up at 3, 9, 24mo or PET/CT, or biopsy
High Risk Patient (eg. smoking history or history of malignancy)
≤ 4mm 12 mo; if no change - stop
4-6mm 6-12mo; no change - follow-up at 18-24 mo
6-8mm 3-6mo; no change - follow-up at 18-24 mo
> 8mm CT follow-up at 3, 9, 24mo or PET/CT, or biopsy
Post-primary TB:
Focal patchy airspace disease “cotton wool” shadows, cavitation, fibrosis, nodal calcification, and flecks of caseous material. These occur most commonly in the posterior segments of the upper lobes, and superior segments of the lower lobes.
Pulmonary hemorrhage:
Blood fills the bronchi and eventually the alveoli.
Has an appearance like that of other airspace filling processes (pneumonia, edema) which have opacity often with air bronchograms.
Caused by trauma, Goodpastrue’s syndrome, bleeding disorders, high altitude, and mitral stenosis.
Notable in that it may clear more quickly than other alveolar densities such as pneumonia.
Pulmonary Embolism
Even though pulmonary embolism is the third most common cause of sudden death, it continues to be underdiagnosed in the intensive care setting. The clinical manifestations of pulmonary embolism are varied. They range from completely silent embolization to sudden death. Symptoms of dyspnea, tachypnea, hemoptysis, hypoxemia, and pleuritic chest pain have been attributed to pulmonary embolism but are neither sensitive nor specific. Indeed, the most valuable indicators of pulmonary embolism are a history of risk factors and or a previous embolic event. Many different medical and surgical conditions are associated with increased risk of pulmonary embolization, including immobilization, trauma, surgery, shock, obesity, pregnancy, polycythemia vera, and antithrombin-III deficiency. The pathophysiology of pulmonary embolism consists of both hemodynamic and respiratory embarrassment. Approximately 90% of pulmonary embolisms are the result of venous thrombosis in the lower extremities. Hemodynamic consequences occur when more than half the cross sectional area of the pulmonary vascular bed is occluded. This situation leads to pulmonary hypertension and in the acute setting right heart failure. Increased alveolar dead space (a result of ventilated but underperfused lung) leads to hypoxemia and respiratory failure. Pulmonary infarction is a rare consequence of pulmonary embolism in patients without concommitent compromise of the bronchial circulation. Generally, infarctions are hemorrhagic and located in the lower lobes.
Radiographic Findings in Pulmonary Embolism (PE)
Due to its relative lack of sensitivity, the chest x-ray in patients with suspected pulmonary embolism is usually relegated to the role of ruling out other disorders which may have a similar clinical presentation. The chest x-ray is also very useful when interpreting ventilation-perfusion scans. Though the majority of patients with pulmonary embolism in retrospect do have abnormalities on the chest x-ray, findings are usually too non-specific to be of diagnostic value. Without infarction there are few chest film signs of pulmonary emboli. These include discoid atelectasis, elevation of the hemidiaphragm, enlargement of the main pulmonary artery into what has been described as the shape of a “sausage” or a “knuckle” (Palla’s sign), and pulmonary oligemia beyond the point of occlusion (Westermark’s sign). Occasionally, pulmonary embolisms will cause infarction causing a unique constellation of radiographic signs. Multifocal consolidation of the affected lung may occur in 12 to 24 hours following the embolic event. A consolidation which begins at the pleural surface and is rounded centrally is called a Hamptom’s Hump. These types of consolidation differ from pneumonia in that they lack air bronchograms. Up to 50% of patients with pulmonary embolism will also have ipsilateral or bilateral pulmonary effusions, although these are certainly nonspecific findings. Nevertheless, it is unusual for pulmonary infarctions to be diagnosed by chest radiography although infarctions are known to occur much more frequently. Presumably infarcts are confused with or indistinguishable from atelectasis or pneumonia. Despite the low sensitivity of these signs, the chest radiograph remains an important first step in the diagnosis of pulmonary embolism, primarily to exclude other causes of hypoxemia and to aid in the interpretation of the ventilation/perfusion scan.
May see: Westermark’s sign (oligemia in area of involvement), increased size of a hilum (caused by thrombus impaction), atelectasis with elevation of hemidiaphragm and linear or disk shaped densities, pleural effusion, consolidation, and Hampton’s hump (rounded opacity). In the case of pulmonary infarctions, the main radiographic feature is multifocal consolidation at the pleural base in the lower lungs.
Several other important modalities are used when investigating possible PE. These modalities are venous ultrasound, V/Q scan, pulmonary arteriogram, and CT angiogram (CTA). Remember, if the CXR of a patient with hypoxia is normal you should consider PE.
The workup of suspected PE can be divided into two populations. In the inpatient setting a CTPA will likely be more definitive than a V/Q scan, as it may disclose other causes of hypoxia not shown on CXR. If the patient has leg swelling, a venous ultrasound of the leg veins should be done to exclude DVT. In the outpatient setting a V/Q scan should be the first test and will less likely be indeterminate than in the inpatient setting. There is also a lower radiation dose for V/Q scans than for CTPA. If these studies are inconclusive a pulmonary arteriogram is the definitive, but more invasive test.

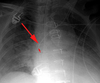
Pneumothorax
Defined as air inside the thoracic cavity but outside the lung (pleural space).
Some causes of spontaneous PTX are; idiopathic, asthma, COPD, pulmonary infection, neoplasm, Marfan’s syndrome, and smoking cocaine. However, most pneumothoraces are iatrogenic and caused by a physician during surgery or central line placement. Trauma, such as a motor vehicle accident is another important cause. Pneumothorax is a common complication of invasive procedures such as central line placement, especially in the mechanically ventilated patient. Barotrauma also can lead to pneumothorax, complicating the intubated patient’s medical course. The air may also arrive at the intrapleural space by rupture of alveoli (blebs), extension of a pneumomediastinum, or communication with extrathoracic air following trauma or surgery.
A tension PTX is a type of PTX in which air enters the pleural cavity and is trapped during expiration usually by some type of ball valve-like mechanism. This leads to a buildup of air increasing intrathoracic pressure. Eventually the pressure buildup is large enough to collapse the lung and shift the mediastinum away from the tension PTX.
On CXR, a PTX appears as air without lung markings in the least dependant part of the chest. Generally, the air is found peripheral to the white line of the pleura. In an upright film this is most likely seen in the apices. A PTX is best demonstrated by an expiration film. It can be difficult to see when the patient is in a supine position. In this position, air rises to the medial aspect of the lung and may be seen as a lucency along the mediastinum. It may also collect in the inferior sulci causing a deep sulcus sign.
A hydropneumothorax is both air and fluid in the pleural space. It is characterized by an air-fluid level on an upright or decubitus film in a patient with a pneumothorax.
Some causes of a hydropneumothorax are trauma, thoracentesis, surgery, ruptured esophagus, and empyema.
Px: Hypotension

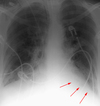
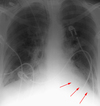
Apicolateral pneumothorax
Appears as a thin, white pleural line with no lung markings beyond. The presence of lung markings beyond this line, though, does not exclude pneumothorax. This is especially true in the patient with parenchymal disease which may alter the compliance of affected lobes, making their collapse more difficult to detect radiographically. Parenchymal disease may also make visualization of the pleural line more difficult or impossible.

Pneumothorax in the Supine Patient
In the supine patient, intrapleural air rises anteriorly and medially, often making the diagnosis of pneumothorax difficult. The anteromedial and subpulmonary locations are the initial areas of air collection in the supine patient. An apical pneumothorax in a supine patient is a sign that a large volume of air is present. Subpulmonic pneumothorax occurs when air accumulates between the base of the lung and the diaphragm. Anterolateral air may increase the radiolucency at the costophrenic sulcus. This is called the deep sulcus sign. Other signs of subpulmonic pneumothorax include a hyperlucent upper quadrant with visualization of the superior surface of the diaphragm and visualization of the inferior vena cava.

Subpulmonary Pneumothorax
Occasionally, a posterior subpulmonary pneumothorax will result in visualization of the more superior anterior diaphragmatic surface and the inferior posterior diaphragmatic surface, resulting in the double-diaphragm sign.

Anteromedial Pneumothorax
Anteromedial pneumothoraces are differentiated into those which are superior or inferior to the pulmonary hilum. A superior anteromedial pneumothorax may result in visualization of the superior vena cava or azygos vein on the right. An inferior anteromedial pneumothorax may be evidenced by delineation of the heart border and a lucent cardiophrenic sulcus. This is the key sign of a pneumothorax as this is the highest point in the supine patient, where the air will accumulate first.

Tension Pneumothorax
Mediastinal shift is usually seen in a tension pneumothorax, but the use of PEEP may prevent this from occurring. The most reliable sign of tension pneumothorax is depression of a hemidiaphragm. Other signs of tension pneumothorax include shifting of the heart border, the superior vena cava, and the inferior vena cava. The shifting of these structures can lead to decreased venous return.
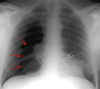
Pneumomediastinum
In the intubated patient the most likely source of air in the mediastinum is pulmonary interstitial air dissecting centripetally. Air in the mediastinum may also originate from tracheobronchial injury or air dissecting through fascial planes from the retroperitoneum. A sudden increase in thoracic pressures (e.g. blunt trauma) may also cause alveolar rupture and consequently pneumomediastinum.
Findings include; streaky lucencies over the mediastinum that extend into the neck, and elevation of the parietal pleura along the mediastinal borders.
Pneumomediastinum often dissects up into the neck. This helps to distinguish it from pneumopericardium that, unlike pneumomediastinum, can extend inferior to the heart.
Causes of pneumomediastinum include; asthma, surgery (post-op complication), traumatic tracheobronchial rupture, abrupt changes in intrathoracic pressure (vomiting, coughing, exercise, parturition), ruptured esophagus, barotrauma, and smoking crack cocaine.
Pneumomediastinum should be distinguished from pneumopericardium and pneumothorax. In pneumopericardium, air can be present underneath the heart, but does not enter the neck.
Continuois diaphram sign
Pneumomediastinum generally will not develop clinical manisfestations. However, a retrosternal crunch is sometimes auscultated (Hamman’s crunch).
Pneumomediastinum rarely causes tension pericardium due to the compressibility of air and the fact that rarely is the pneumomediastinum non-communicating tension due to air is rare. Pneumomediastinum may cause pneumothorax (the reverse is not true) or pneumoperitoneum.