dental materials 93 Flashcards
(310 cards)
force in
mg
m =
mass (kg)
g =
gravitational acceleration (10ms-2)
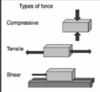
compressive strength
resistance to breaking from a force acting to reduce its size
tensile strength
resistance to breaking from a force acting to elongate
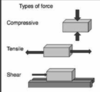
shear strength
resistance of a material to moving along an axis which is parallel to the forces direction
strain
change in length / original length
(L1 - L0) /L0
given as a ration or %

Young’s Modulus =
Stress /strain
F/A or (L1-L0)/L0
given in MPa
assess how rigid a material is

opposite of rigid is
flexible
fracture
large force causes a catastrophic destruction of materials structure
hardness
ability of surface to resist indenetation (KHN)
abrasion
material surface removal due to grinding
abrasion resistance
ability to withstand layers being removed compromising surface integrity
grinding along opposing tooth surface
fatigue
repititive ‘small’ stresses cause material fracture
creep
gradual dimensional change due to repetitive small forces (amalgam when it creeps above margins - standing proud then fracture)
deformation
an applied force may cause a permanent change in materials dimensions (not fracture it)
elasticity
impression materials - strain and recoverery
de-bond
applied forces sufficient to break material tooth bond by shear forces (ortho appliances)
impact
large sudden forces causes fracture - curve of upper dentures to accomodate palate maean that they are liable to snap
bonding to enamel
hetergenous structure (5% organic, 95% inorganic)
‘dry’
acid etch technique - remove cores of enamel prism leaving just peripheral enamel (creates pores for resin)
bonding to enamel is simple
bonding to dentine
dentine composition - 20% organic (collagen), 70% inorganic (hydroxyapatite), 10% water
fluid from pulp flows up dentine base making the surface wet
dentine varies - aged dentine more mineralised, pulpal dentine has increased moisture content
requirements of dentine bonding agent DBA
flowability
intimate contact with dentine surface
low viscosity
adhesion to substrate - mechanical, chemical, van der waals
smear layer is
pulp, dentine, bacterial debris plug dentine holes
what to do with smear layer
has to be removed by acid conditioning to either dissolve or solubilise the plugs
expose the tubules to create pores for resin