Digestion and Absorption of Lipids & Proteins Flashcards
(48 cards)
Dietary lipid intake
- 25-160g/day
- Mostly TAG
- Phospholipid
- Cholesterol ester and free cholesterol
What are the essential lipids
- Fat soluble - A, D, E, K
- Essential FAs - linoleic acid, linolenic, arachidonic acid
- < 6 g of fat is eliminated/day (bacterial cells and cell debris)
What lipids are soluble in water
How are they transported
- Short chain FAs (< 10 C) are soluble in water
- Absorbed by passive diffusion
- Transported down their conc gradient and into portal blood
- Rapid process
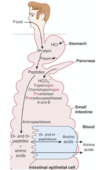
How are non-soluble lipids transported
- Chyme in lumen
- Cell interior
- IS fluid
- Lymph
- Blood
Fats that are water soluble
- Lipases
- Phospholipases
- Cholesterol esterases
Composition of Triglyceride

Pathway of digestion of Triacylglycerol
- Begins in the stomach (lingual lipase has a minor role)
- Gastric lipase
- Pancreatic lipase - yields 2 monoacylglycerol and FAs
- CHOLESTEROL esterases in pancreatic juice - yields free cholesterol and FAs
- PHOSPHOLIPID A in pancreatic juice (zymogen) - activated in SI by trypsin - acts on a variety of phospholipids
What are acid stable lipases
What is their role
- Lingual and gastric
- Play a major role in neonates
- Digest milk which is a major source of short and medium chain FAs ( < 12 C)
- Major source of calories
- Important in pancreatic insufficiency - cystic fibrosis

Physical emulsification of fats
- Change in physical nature begins in stomach
- Core body temp helps liquify lipids
- Peristaltic movements aid in emulsion formation
Chemical emulsification of fats
- Acid-stable salivary and gastric lipases assist
- Initial hydrolysis is slow
- Relatively small lipid-water interface
- Once hydrolysis begins, water-immiscible TAGs are degraded to FAs and they act as surfactants
- FAs confre hydrophilic surface to lipid droplets and break them down into smaller particles, increasing the lipid-water interface, facilitating rapid hydrolysis
- Lipid phase becomes dispersed throughout the aqueous phase as an emulsion
Benefits of the emulsification of lipid
- Increase SA so that digestive enzymes can work on them
- Detergent properties of bile salts - derivatives of cholesterol
- Mechanical mixing due to peristalsis
What is the most abundant bile salt in humans
Glycocholate
Sequence of events in fat digestion
- TAG - pancreatic lipase yields 2 monoacylglycerol and free FAs
- Colipase (secreted as procolipase) binds lipase and anchors it to the aqueous surface
- Cholesteryl ester - cholesteryl esterase - activity greatly increased in presence of bile salts

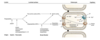
Schematic of digestion of lipid

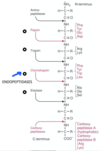
Phospholipid degradation
PROENZYME OF PHOSPHOLIPASE A
- Activated by trypsin
- Requires bile salts for optimum activity
- Removes 1 FA from C 2 to form lysophospholipid
- Next FA at C 1 can be removed to form glycerylphosphoryl base
Control of lipid digestion
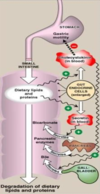
How are lipids absorbed
BY ENTEROCYTE
- Free FA, free cholesterol and 2 monoacylglycerol
- With bile salts form mixed micelles
- hydrophobic grps on inside
- Hydrophilic grps on outside
- mix with unstirred water layer
- Short and medium chain FAs DO NOT require formation of micelles for transport
How are triacylglycerols and cholesteryl esters re-synthesised
- Mixture of lipids migrate to ER
- Remake complex lipids
- FAs are converted to fatty acyl CoA
- Fatty acyl CoA synthetase
- 2 monoacylglycerols converted to triacylglycerol

Secretion of lipids from enterocytes
- TG and CE are VERY hydrophobic
- Need to be re-packaged
- lipid droplets are surrounded by a thin layer composed of phospholipids, unesterified cholesterol and a protein molecule apolipoprotein B-48
- The protein component, apolipoprotein B-48 is essential for the final release of chylomicrons from the enterocyte - requires protein synthesis
- Sent to lymph
2 causes of fat malabsorption
PANCREATIC FAILURE
- bulky, fatty, floating stools that contain undigested triglycerides - steatorrhea
- Deficiencies of fat-soluble vitamins can occur
LACK OF BILE SALTS
- Most of the “fat” in the stools consists of unabsorbed FAs, monoglycerides and diglycerides
- Fat malabsorption can be treated effectively
- Pancreatic enzymes and bile salts both available in tablet form
- Used routinely in patients with pancreatic insufficiency or biliary disease
Fat malabsorption

Total daily protein load
- 70 - 100g of dietary protein
- 30 200g of endogenous proteins
How much protein is lost in digestion
6-12g - digestion is very efficient
Very little absorption of…..
EXCEPT
Very little absorption of intact protein
EXCEPTION - newborns can take up maternal antibodies