Elements Compounds and Mixtures Flashcards
(24 cards)
Name the parts of an atom
proton
neutron
electron

what is the charge of an electron
negative charge

what is the relative mass of an electron
0

what is the relative mass of a neutron
1

what is the relative mass of a proton
1

what is the charge of a proton
postitve charge

what is the charge of a neutron
neutral charge

where are the protons and neutrons found in an atom
nucleus

where are the electrons found in an atom
electron shells

what is the mass number of an element
number of protons + number of neutrons

what is the atomic number of an element
number of protons

how do you find the number of neutrons of lithium

7-3=4
mass number - proton number
how do you know that an atom is neutral in charge

number of protons equals number of electrons

descire how many electrons arwe found in the first four shells of a atom
2 in the first
8 in the second
8 in the third
2 in the fourth
an element is
a substance made of one type of atom

a compound is
a type of substance that ius chemically with two oor more different types of atoms

a molecule is
a group of atoms chemically bonded

a pure substance is
matter is pure when all the substances within it are the same
example- Boxes X, Y and Z are pure substances

a mixture is
2 or more different substances that are not chemically bonded
Example: Box D is a mixture- other boxes are pure substances

Which box/es contains a pure element?
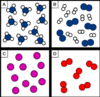
Box C and D only

Which box/es contains a mixture of elements?

Box B only

Which box/es contain molecules?
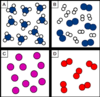
Box A, B and D only
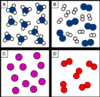
Which box/es represents a mixture?

Box B only
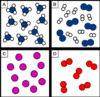
Which box represents a pure substance?

Box A, C and D



