exam 3 Flashcards
(23 cards)
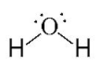
Tetra Hedral Bent
104.5

Linear Linear
180

Octahedral Octahedral
90

Trigonal Bypyrimadil Seesaw
90

Trigonal Bypyrimidal T Shaped
90

Trigonal Bypyrimidal Linear
180
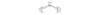
Trigonal Planar Bent
120
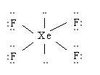
Octahedral Square Planar
90

Tetrahedral Tetrahedral
109.5

Trigonal bypyrimidal Trigonal Bypyrimidal
120
109.5

Tetrahedral Trigonal Pyrimidal
107

Trigonal Planar Trigonal Planar
122

Octahedral Square Pyrimidal
90
Atomic Radii increase which way on the columns of the periodic table?
Down
Atomic Radii increase which way on the rows of the periodic table?
Left
Which group has the lowest ionization energy?
1A
which group has the highest ionization energy?
Noble Gases
Ionization Energy does what as you move down a row?
Decreases
Which group has the highest electron affinity?
7A
which group have the lowest electron affinities?
2A and 8A
Ionic bonds form between elements which have what?
A small ionization energy and a high electron affinity
When is lattice energy a large value?
When the atoms radii are small, and when there charges are large.
Electronegativity increases in what direction?
Left to Right
and Bottom To Top


