Immunology - Exam 3 Flashcards
(57 cards)
Describe imunological tolerance.
Immunological Tolerance
- IT is specific UNRESPONSIVENESS to an Ag.
- SELF‐TOLERANCE - All individuals are tolerant to self‐Ags.
- AUTOIMMUNITY results from breakdown of self‐tolerance.
- The NEGATIVE SELECTION of self‐reactive T lymphocytes in the thymus is NOT perfect.
- There is a LOW LEVEL of physiological auto‐reactivity that is crucial to normal immune function.
- The challenge is to understand how it becomes a PATHOLOGIC PROCESS and how T cells and B cells recognize self and contribute to tissue injury.
Compare central vs peripheral intollerance.
Immunological Intollerance - Central vs Peripheral
- Central:
- Induced in immature self‐reactive lymphocytes in the primary lymphoid organs.
- Ensures that mature lymphocytes are NOT REACTIVE to self Ags.
- Immature lymphocytes specific for self Ags may encounter these Ags in the generative (central) lymphoid organs and are either:
- Deleted
- Change BCR specificity
- Develop into Treg cells.
- Peripheral:
- Induced in mature self‐reactive lymphocytes in peripheral sites.
- Needed to prevent activation of these potentially dangerous lymphocytes in the tissues.
- Mature self‐reactive lymphocytes in peripheral tissues may be either:
- Inactivated (anergy)
- Deleted (apoptosis)
- Suppressed by the Treg cells

Describe central T cell tollerance.
Central T Cell Tolerance
- Takes place in THE THYMUS.
- Thymocytes undergo a maturation and selection process.
- Nonfunctional thymocytes showing NO AFFINITY at all undergo apoptosis.
- STRONGLY SELF‐REACTIVE THYMOCYTES - as determined by interactions with MHC‐self peptide complexes - are also deleted.
- Only thymocytes that are activated by MHC‐ self peptide complexes BELOW A CERTAIN THRESHOLD are positively selected and migrate into the periphery as mature T cells.
- Most of these thymic emigrants develop into effector CD4+ and CD8+ T cells, and mediate both cell‐mediated and humoral (Ab‐mediated) immune responses.
- A SMALL PERCENTAGE OF T CELLS that emigrate from the thymus express FOXP3 and develop into natural CD4+CD25+CTLA4+ Treg cells.
Describe central B cell tolerance.
Central B Cell Tolerance
- CLONAL DELETION and ANERGY were major mechanisms mediating central tolerance of developing autoreactive B cells, resulting in the elimination of autoreactive clones, and preventing immune responses against self.
- When an immature B cell reacts with a self‐antigen with HIGH AVIDITY, such as a highly expressed membrane‐bound protein, it undergoes apoptosis within 2–3 d.
- In contrast, LOW AVIDITY interactions of B cells with self‐antigens induce unresponsiveness to subsequent stimulation or anergy but allowed for migration into peripheral compartment. The anergic B cells fail to enter follicle and have reduced life‐span.
- However, clonal deletion and anergy are not the only modes of selection against autoreactive immature B cells, but there operates another system, namely, RECEPTOR EDITING.
- Autoreactive immature B cells reactivated their Ig gene rearrangement program at the Ig light chain loci resulting in the expression of a new light chain that paired with the existing H chain to form a non‐autoreactive BCR, an event that promoted the selection of these edited B cells into the periphery.

Describe BCR editing.
BCR Editing
- Precursor (pre)‐B cells, which already express rearranged IgH chains recombine the locus that encodes IgL chain, yielding a lymphocyte with an autoreactive antigen receptor.
- BCR signaling promotes developmental arrest and continued recombination.
- Receptor editing of the IgL chain leads to expression of a distinct IgL chain, generating cell‐surface immunoglobulin that lacks self‐reactivity

Describe deletion of self-reactive lymphocytes.
Deletion of Self-Reactive Lymphocytes

Describe the role of Treg cells in peripheral tolerance.
Peripheral Tolerance - Role of Treg Cells
- Treg cells are key mediators of peripheral tolerance.
- Treg cells may inhibit T cell activation by APCs and inhibit T‐cell differentiation into CTLs.
- Treg cells may prevent T cells from providing help to B cells in the production of Abs.
- FOXP3+ Treg cells can also be generated from peripheral T cells (not shown).

Compare natural and inducible Treg cells.
Natural vs. Inducible Treg Cells
- The development and survival of these regulatory T cells require IL-2 and FoxP3.
- In peripheral tissues, Treg cells suppress the activation of self-reactive lymphocytes.

Describe induced Treg cells.
Induced Treg Cells
- Differentiate in the periphery.
- In addition to the natural Treg cells which differentiate in the thymus, mature T cells OUTSIDE THE THYMUS can also acquire Treg phenotype and function.
- These are called induced Treg cells (iTreg cells).
- FoxP3 EXPRESSION can be induced in naive CD4+ cells in vitro by antigen recognition in the presence of TGF‐β.
- There is a close developmental RELATIONSHIP between iTregs and Th17 cells.
- Ag recognition in the presence of TGF‐β induces FoxP3 expression if IL‐6 is NOT present.
- In contrast, Ag recognition in the presence of TGF‐β + IL‐6 prevents FoxP3 expression, induces expression of the retinoic acid receptor (RAR) related orphan nuclear receptor RORγt expression and therefore, Th17 cell DIFFERENTIATION.

Describe peripheral B cell tolerance.
Peripheral B Cell Tolerance
- Mature B cells that recognize self Ag in peripheral tissues in the absence of specific Th cells may be rendered functionally UNRESPONSIVE or die by APOPTOSIS.
- The CD22 inhibitory receptor is phosphorylated by Lyn and then recruits SHP‐1 tyrosine phosphatase attenuating BCR signaling.
- Therefore, DEFECTS in Lyn tyrosine kinase, SHP‐1 tyrosine phosphatase, and the CD22 inhibitory receptor lead to AUTOIMMUNITY.

Describe the mutations breaking tolerance.
Mutations Breaking Tolerance
- Incomplete induction of tolerance in the thymus (AIRE deficiency causes Autoimmune Polyendocrine Syndrome).
- Impaired production of regulatory T cells (FoxP3 deficiency causes IPEX syndrome).
- DECREASED CLEARANCE and impaired tolerance induction by apoptotic cells (complement deficiency of C1q and C4).
- ALTERED IMMUNE SIGNALING thresholds (CTLA‐4 polymorphisms).
- Loss of Self Tolerance Leads to Autoimmunity.

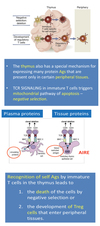
Describe AIRE (AutoImmune Regulator) in central tolerance.
Central Tolerance - AIRE (AutoImmune Regulator)
- The NEGATIVE SELECTION of T cells in the thymus is necessary for the maintenance of self tolerance.
- Medullary THYMIC EPITHELIAL CELLS have a key function as APCs.
- They EXPRESS a large number of SELF‐Ags that are presented to developing T cells.
- MUTATIONS in AIRE (autoimmune regulator ) protein cause a breakdown of central tolerance.
- AIRE has been proposed to function as a TRANSCRIPTION FACTOR.
- Mutation in AIRE is associated with DECREASED EXPRESSION of self‐Ags in the thymus.

Describe how aberrant expression of AIRE leads to autoimmunity.
Autoimmunity - Aberrant Expression of AIRE
- The AIRE regulates the expression of tissue‐restricted Ags (TRAs).
- Peptides derived from these Ags are displayed on the Medullary Thymic Epithelial Cells.
- Ags are recognized by immature Ag‐ specific T cells, leading to the deletion of self‐reactive T cells.
- In the absence of functional AIRE, these self‐reactive T cells are not eliminated and they can enter tissues where the Ags continue to be produced and cause injury.

Describe the outcomes of Ag-dependent T cell activation.
Ag-Dependent T Cell Activation - Outcomes

Describe the role of CTLA4 (Cytotoxic T-Lymphocyte Antigen 4) in peripheral tolerance.
Peripheral Tolerance - Role of CTLA4 (Cytotoxic T-Lymphocyte Antigen 4)
- Upon Ag ENCOUNTER, individual populations of T cells undergo expansion and later contraction after the elimination of Ag.
- T cell activation is regulated by members of the B7‐CD28 family of COSTIMULATORY MOLECULES.
- CTLA4 (Cytotoxic T‐Lymphocyte Antigen 4) is a homolog of CD28.
- CTLA4 is an INHIBITORY RECEPTOR.
- CTLA4 provides signals that terminate immune responses and maintain self‐tolerance.
Describe the funcitons of CTLA-4.
CTLA-4 Functions
- UNCONTROLLED LYMPHOCYTE ACTIVATION with massively enlarged LNs and spleen and fatal multi-organ lymphocytic infiltrates is seen in CTLA-4 KO mice.
- BLOCKING of CTLA-4 with Abs also enhances autoimmune diseases in animal models.
- POLYMORPHISMS in the CTLA-4 are associated with several autoimmune diseases in humans, including type 1 diabetes and Graves’ disease.
- CTLA-4 has two important properties:
- CTLA-4 expression is low on resting T cells until the cells are activated by Ag.
- Once expressed CTLA-4, terminates continuing activation of these responding T cells.
- CTLA-4 is expressed on REGULATORY T cells and mediates the suppressive function of these cells by inhibiting the activation of naive T cells.
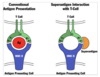
Describe the MOA of CTLA-4.
CTLA-4 - Mechanism of Action
- CELL-INTRINSIC ACTION:
- Engagement of CTLA-4 on a T cell may deliver inhibitory signals that terminate further activation of that cell.
- CELL-EXTRINSIC ACTION:
- CTLA-4 on Treg cells or responding T cells binds to B7 molecules on APCs or makes unavailable to CD28.
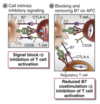
Describe Treg cells and their role in regulating T cell responses.
Treg Cells
- Treg cells develop in THE THYMUS.
- Treg cells are POSITIVELY SELECTED in the thymus via strong TCR interactions with self‐Ags.
- After recognition of self‐Ags they are NOT ELIMINATED by apoptosis.
- Treg cells are able to produce ANTI‐APOPTOTIC MOLECULES which protect them from negative selection in the thymus.
- The generation of some Treg cells requires the TGF‐β.
- Treg cells express FOXP3 transcriptional factor and are CD4+CD25+ positive.
- Treg cells typically express high levels of CTLA‐4.
- CYTOKINE IL‐2 is critical for survival and functional competence of Treg cells.
- Treg cells are endogenous LONG‐LIVED populations of self‐Ag‐specific T cells.
- Treg cells serve to prevent potentially AUTOIMMUNE REACTIONS.

Describe TGF-β.
Transforming Growth Factor - β
- INHIBITS the proliferation and effector functions of T cells.
- INHIBITS development of Th1 and Th2 subsets but PROMOTES Th17 in cooperation with IL‐1 and IL‐6.
- INHIBITS activation of M1 macrophages.
- REGULATES the differentiation of induced FoxP3+ Treg cells.
- STIMULATES production of IgA by inducing B cells to switch to this isotype.
- PROMOTES tissue repair after local immune and inflammatory reactions subside stimulating collagen synthesis and matrix‐modifying enzyme production by macrophages and fibroblasts.
Describe autoimmunity.
Autoimmunity
- About 5% or ~ 12‐15 million people from AUTOIMMUNE DISEASES in the US alone suffer.
- There are 60‐70 diverse autoimmune diseases which affect various tissues of the human body.
- There is NO known CURE or clear UNDERSTANDING the cause for any of autoimmune conditions.
- Most autoimmune diseases are treated symptomatically.
- The autoimmune diseases bring the PARADOX proposition that “the body both is and is not itself”.
Describe autoimmunity in chronic disease.
Autoimmunity - Chronic Disease
- There is NO FUNDAMENTAL DIFFERENCE between the structure of self auto‐Ags and non‐self Ags because Ags are all proteins composed by the same amino acids.
- PATHOLOGIC immune RESPONSE against self Ags often clinically manifested as “immune‐mediated inflammatory diseases”.
- CAUSED BY the activation of T cells and/or B cells in the absence of an ongoing infection or other discernible cause.
- A result of a HYPERSENSITIVE IMMUNE SYSTEM that causes one’s own immune system to attack the self.
Describe the prevention of autoimmunity.
Autoimmunity - Prevention
- T cells that are physically separated from their specific Ag (the BBB) cannot become activated, a process termed immunologic ignorance.
- T cells that express the Fas (CD95) can receive their signals from cells that express FasL and undergo apoptosis, a process known as deletion.
- CTLA4 (CD152) that binds CD80 on APC and inhibits T cells activation.
- Regulatory T cells can inhibit through the production of inhibitory cytokines such as IL-10 and TGFβ.

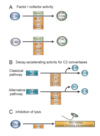
Describe the factors determining Ag response vs tolerance.
Factors Determining Ag Response vs Tolerance

Describe the mechanisms of autoimmunity.
Autoimmunity - Postulated Mechanisms
- Various genetic loci may confer SUSCEPTIBILITY TO AUTOIMMUNITY, in part by influencing the maintenance of self‐tolerance.
- Environmental triggers, such as infections and other inflammatory stimuli, promote the influx of lymphocytes into tissues and the activation of self‐reactive T cells, resulting in tissue injury.