Immunology Midterm #2 Flashcards
(84 cards)
What is humoral immunity?
B cell immune factors which are dissolved in the extracellular fluids (complements and antibodies)
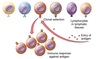
What is clonal selection?
Rapid reproduction of a single clone (single b cell with a specific epitope)
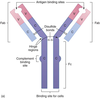
What is an antibody?
are B cell receptors (BCRs) which are secreted in the blood. aka immmunoglobulins
What is affinity? How does it relate to the antigen binding sites?
Affinity is the strength which as antibody or other receptor binds to an epitope. Certain antigens bind better to some sites than other. However, an imperfect fit can still cause a reaction and cause Abs to be released
What determines the shape of the antigen binding site?
VDJ recombination
What are the differences between V and C segments?
V - variable segment - determines which antigen binds to the epitope site as a result of VDJ recombination
C - constant segment - defines the function of the antibody
When is a receptor not an immunoglobulin?
When it doesn’t activate the immune system ie blood glucose receptors
Is a T cell receptor an immunogloblulin? an antibody?
Immunoglobluin: yes
Antibody - no
What enzymes can split an antibody? What are the functions of the Fab and Fc?
Split by proteases such as papain protease
Fab (Fragment of antigen binding) - antigen binding, neutralization, agglutination
Fc (constant fragment) - opsonization, complement receptor binding
What are the events of Ab production and specification?
- VJD recombination produces a IgM antibody with a specific antigen binding site
- The B cell is exposed to an antigen
- The C section undergoes recombination, depending on what is required
- Clonal selection expands required population
What is class switching?
Changing the function of an antibody by switching the Fc.
Done by a process of looping and cutting until the correct Ab is at the front

What is IgM? What is the shape? Function? Special Characteristics?
Immunoglobulin prior to C recombination.
As a monomer - interacts with APCs or pathogens
As a pentadimer - secreted in blood. Compliment fixation and agglutination

Provide a summary of the different antibodies and the # of binding sites,
if it crosses the placenta
fixes complements
binds to what cells
function

How does IgD compare to IgM?
Thought to have similar functions to IgM - agglutination and compliment fixation
What is IgA? What is the shape? Function? Special Characteristics? How many classes?
Antibody found in secretions
Antibody found in seromucous secretions (saliva, breastmilk, colostrum, genitourinary). Coated with glycoprotein which protects mucous membrane from antigen attachment
Travels through the epithelium - gains epethilial receptor to stabilize IgA
Shape - dimer
Classes: 2

What is IgG? What is the shape? Function? Special Characteristics? Classes?
80% of circulating antibodies. Monomers. Long lived. 4 classes
Function: Neutralization, opsonization, compliment fixation. Not as great with agglutination
Features: Can cross placenta to give fetus passive immunity, Has memory
What is IgE? What is the shape? Function? Special Characteristics?
Scarce Ig. Only elevated in certain conditions (parasitic infections and allergies). Attaches to eosinophils and basophils
How do antibodies promote pathogen killing?
ANtibodies will attach to specific cellular receptors which dictates what will happen
ie IgG will bind to a Fcy receptor and cause opsonization
What are the effects of antibodies of agglutination? What is a clinical practice based on this?
Antibodies with multiple Fab sites clump antigens together to make for easy digestion (ie kitty litter) (IgM has 10 Fab)
Blood typing: antibodies for a certain blood type are placed in a blood sample. If the antibodies match the blood type, the sample with be clear with visible clumps
Can also clump precipitates from a solution,
What are the effects of antibodies of neutralization? Clinical relevance?
ANtibodies block the attachment of microorganisms to susceptible tissues and cells (ie IgA)
HIVmab B12 blocked the HIV virus from entering CD4 cells. Prevent exotoxins from entering cell by preventing binding (antitoxins)

What are the effects of antibodies on oponization?
ABs can activate the compliment system, or can improve phagocytic abilities of cells (macrophages) by coating the macrophage with Abs (IgG)
What is antibody dependent cell mediated cytotoxicity? Examples?
Part of the normal immune response but can cause damage to tissues when prolonged.
Ie - opsonization - binds to the Fc of pahgocytes
Frustrated phagocytosis - target is too large to engulf, so digestive enzymes are secreted which can cause inflammation of normal tissues
Eosinophil response - IgE bound cells secrete toxins and toxic factors
What are the effects of Ab on complement activation?
Ab-Ag complexes activates the complement factors by the classical pathway which results in chemotaxis, inflammation, opsonization and the formation of MAC
The antibodies themselves do not induce chemotaxis, but the complement factors (C3a, C5a)
What do Tc cells use to MHCI? What protein binds to MHC2
Tc use CD8 to bind to MH!
CD4 binds to MH2


