Intro Flashcards
(78 cards)
Nearly 100% of human cancer has ____ pathway inactivation, >90% has ____ inactivation
Nearly 100% of human cancer has p16INK4a-Rb pathway inactivation, >90% has p53 inactivation
What are 4 aspects of dysregulation present in the cellular processes of cancer?
I. Inappropriate proliferation
II. Resistance to differentiation and apoptosis
III. Genomic Instability
IV. Ability to grow where it ought not (i.e. “malignant growth”)
Protooncogene:
Highly conserved eukaryotic genes, important in cellular growth and development, which can become oncogenes either by over/under expression or by mutation
Cellular Oncogenes:
(c-onc); cellular genes involved in the development and/or maintenance of the malignant phenotype
Viral oncogenes:
(v-onc); viral genes which are able to transform cells
Oncogenic mechanisms (4):
•Growth factors
- Mutated ras induces expression of normal growth factor genes (TGF-a)
- The sis viral oncogene is highly homologous to PDGF, and can stimulate PDGF-R
•Signal transduction
- Her-2/neu amplification in breast carcinoma
•Cell cycle control
•Regulation of gene expression
Give 2 examples of growth factor involvement in oncogenic mechanisms:
–Mutated ras induces expression of normal growth factor genes (TGF-a)
–The sis viral oncogene is highly homologous to PDGF, and can stimulate PDGF-R
Provide an example of oncogene development via signal transduction:
Her-2/neu amplification in breast carcinoma
There are professional tumor suppressor genes (e.g. ____ and ____) that are dispensable for normal development, but serve only to prevent _______.
There are professional tumor suppressor genes (e.g. p16INK4a and p53) that are dispensable for normal development, but serve only to prevent transformation.
Li-Fraumeni Syndrome:
Hereditary predisposition to cancer.
p53 protein normally
- Arrests cell cycle when DNA damage occurs
- Promotes apoptosis in damaged cells
- Most commonly mutated gene in cancer
Persons with syndrome are born with one abnormal copy
- Tumors have mutations at both alleles
- Glioblastomas, leukemias, breast, lung, and pancreatic CA, Wilm, sarcomas

Fill in the empty spaces


FIll in the blanks (shows resistance mechanisms to apoptosis)


Hyperplasia:
Increase in the cell number
Usually associated with an increase in tissue mass (hypertrophy)
Can be physiologic or pathologic
Explain how hyperplasia can be physiologic or pathologic:
Physiologic:
- Hormonal (glandular epithelium in breast during puberty)
- Compensatory (contralateral kidney after nephrectomy)
Pathologic (breeding ground for cancer):
- Endometrial hyperplasia
- Benign prostatic hypertrophy (BPH)
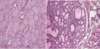
What types of cells are shown in these images?
Hypertrophy:
•Increase in cell size
- Production of new subcellular components (not swelling)
•Can be physiologic or pathologic
Explain how hypertrophy can be physiologic or pathologic:
–Physiologic
- Skeletal muscle
–Pathologic
- Cardiomyocytes and increased afterload (HTN) (although limited proliferation may be possible!)
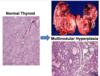
What change is indicated in these images?

hypertrophy
Define metaplasia:
•Change of one differentiated cell type into another differentiated cell type
–Usually an adaptive response
–Usually reversible
List two common examples of metaplasia:
–Smoking and respiratory epithelium
–Barrett esophagus
What change has occurred in these two images?
This is an image of the esophagus.
Left is NORMAL
Right is METAPLASTIC.
Define dysplasia:
Atypical proliferation of cells with:
- Abnormal appearance
- Variation in size and shape (pleomorphism)
- Nuclear enlargement (increased nuclear-to-cytoplasmic ratio; increased N:C)
- Nuclear irregularity
- Dark staining of nuclei (hyperchromasia)
- Disorderly arrangement
- Loss of polarity (disrupted order of expected growth)
- Loss of maturation
- Abnormal location of mitotic figures
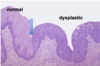
Describe this image:
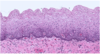
Normal squamous epithelium
What is shown in this image?
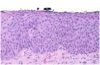
Dysplastic squamous epithelium