Introduction to Pharmacology Flashcards
(31 cards)
what is pharmacology?
study of substances that interact with living sytems through chemical processes: interaction is usually by binding to reg. molecules and activate or inhibit normal body processes
what is a drug?
any substance that interacts with a molecule or protein that plays a reg. role in living systems:
- hormones: endogenous drugs
- *-** poisons
- toxins are poisons of biological origins
what are the 4 main classes of receptor?
which is slowest -> fastest?
- Ion channel
- G-protein activation
- phopshorlyation of tyrosines on key siganlling molecules
- transport to nucleus
FAST - 1—> 4 - SLOW
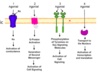
which type of receptor is most common ?
G-protein activation
what is an agonist?
- any drug that binds to a receptor and activates the receptor
- mimics the natural ligand of receptor
- when ligand leaves:
a) usually deactivates receptor & stops effect
b) some agnonists are permenantly activated even after ligand has left (covalent change in receptor)
what is a partial agonist?
what does this mean with dosing?
partial agonist:
- lower affinity to receptor: reduced intrinsic activity
- binds to receptor but doesnt activate to maximal response / gives partial effect
- often prevents other agonists from binding
- much harder to overdose: difficult to get to toxic AEs

what is an inverse agonist?
what do they only work on?
inverse agonist:
- acts on unoccupied receptors to produce effect opposite of agonist: negative efficacy
- shifts to inactive state
- only work on systems that are constitutively active: autonomic NS and histomine systems

what is a pharmacologic antagonist?
what are the two types?
pharmacologic antagonist
- any drugs to binds to a receptor and prevents activation of the receptor
two types:
1. competitive antagonist
- fits into lock but does not activate receptor
- competes with agonist
2. non-competitive antagonist
- binds to a site that is not the activation site (where agonist binds)
- changes conformation of receptor - agonist cant fit
which pharmocological antagonists:
- reduces agonist efficacy?
- reduces agonist potency?
- reduces agonist efficacy: non-competitive antagonist
- reduces agonist potency: competitive antagonist
what is a chemical antagonist?
what is a physiological antagonist? e.g.?
chemical antagonist: binds directly to an agonist preventing it building to receptor or substrate
physiological antagonist: two drugs that have exactly opposite actions via different pathways
- e.g. adrenaline when have allergic reaction (oppposite effect and pathways to histamines)
what type of drug is heparin?
chemical antagonist
- binds to thrombin and inactivates it: stops thrombin to going to clotting cascade
what inactivates heparin if have too much?
(protamine sulfate)
what do drugs need to do to work ?
ADME
Absorption (oral / IV)
Delivery
Metabolism
Elimination
what do most drugs tend to be?
what are their properties?
weak acid or weak base (incomplete dissociation in water)
- less H and negative charges (than strong acid)
- undissociated form of acid / base
- lipid soluble
why is acid / base relationship of drugs important for drug elimination?
explain how ion trapping works with aspirin
(pKa (pH at which drug is completely balanced between un-charged (lipid soluble) and charged (water soluble) form)) = 3. )
aspirin: R-COOH. exists in equilibrium of R-COOH (protonated) to R-COO- H+ (un protonated)
- when take aspirin orally: goes into stomach
- stomach has low pH, more free H+ ions in solution : pushes aspirin equation to R-COOH (uncharged aspirin, fat soluble, protonated form) = absorbed across stomach mucosa
- then moves to blood plasma: pH 7.
- moves to R-COO- + H+ (ionic aspirin, hydrophilic - water soluble) = trapped in blood plasma (where want to be trapped) cant return to stomach.
THEREFORE - NEED TO BE ABSORBED IN THE STOMACH BECAUSE V LITTLE WOULD BE STORED IN AN ALKALI SOLUTION
- this process = ion trapping
- Aspirin and many NSAIDs are weak organic acids that remain in the nonionized form in the strong acidic environment of the gastric lumen and can freely diffuse across the cell membrane. Once across the membrane, the high intracellular pH causes the H+ to dissociate, trapping the negatively charged organic compound in the cell.*

a protonated (less dissociated / proton still there) weak acid is more X soluble?
an un-protonated (more dissociated / proton left) strong acid is more X soluble?
1. lipid soluble (liphophilic)
2. water soluble (hydrophilic)
what happens to a protonated drug in the kidney?
- almost all drugs are filtered by kidneys
- protonated drug in kidney: reabsorbed back into blood
what happens in the kidney when you have a tricyclin antidepressant (TCA) overdose?
- pKa = 9.5 (weak base)
- in the body TCA causes metabolic acidosis
- in blood: sodium bicarbonate dissociates TCA = charged R-NH2 form: lipid soluble
- once in acidic urine: reforms R-NH3 form: water soluble
- excreted

what is specificity and selectivity of a drug?
specificity: range of actions produced by a drug (determined by receptors drug binds to)
selectivity: ability of drug to preferentially produce a specific effect (WHICH RECEPTOR THE DRUG WILL BIND TO AND HOW STRONGLY TO GET AN EFFECT). e.g. if you increase the dose of a drug, it might cause different effects.
for all purposes these are the same thing and often misused
what is drug affinity?
affinity: how tightly drug binds to receptor
what is responsible for selectivity of drug action?
receptors: that receptor decides what internal signalling mechanisms are activated
what can affect the action of a drug?
what are the three sub categories of ^?
- *other drugs**
- allosteric interactions: indirectly affect receptor -> affect efficacy or binding affinity
- allosteric activators; increase activation of receptor
- allosteric inhibitors: decrease activity of the receptor




