Lecture 9 - Immunity to meningitis Flashcards
(26 cards)
What are the 3 mechanisms of complement activation, and what do they all produce?
- Lectin pathway (microbial glycans
- Classical pathway (Antibody)
- Alternative pathway (Deposition of spontaneously formed C3b)
They all generate C3 convertase, which cleaves C3, leaving C3b bound to the microbial surface and C3a is released
When Strep. Pneumoniae colonise the epithelial surface, how does the adaptive immune response occur?
M cells, which sit in the epithelial lining, take up an antigen and present it to dendritic cells which are underlying the epithelial surface. This can then start the adaptive immune response in the lymphoid follicles. This results in antibodies being produced from B cells, and these bind to polymeric immunoglobin receptor and are secreted across the epithelial surface. These Ab’s will be specific for the capsule of the specific organism, this protects us against bacterial invasion
How are antibodies secreted across the epithelium?
They are secreted by polymeric immunoglobulin receptor (PIgR)
These antibodies are serotype specific and protect us from colonisation and invasion
How to bacteria cross epithelium to enter the blood stream? (usually across nasopharynx epithelium)
Bacteria can use reverse transcytosis to translocate across epithelium, after this it can then enter the blood stream by recognising receptors on the basal surface of endothelial cells, which then allow it to move into the blood stream

What are the soluble molecules present in the blood stream for innate immunity?
Collectins
Pentraxins
Complement

What do collectins, pentraxins, complement and antibodies all do?
Opsonise bacteria, which leads to phagocytosis
Collectins, pentraxins and anitbodies all help activate complement

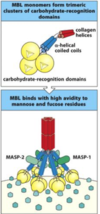
Briefly describe the lectin pathway
It involves collectins which circulate in blood (e.g. mannose binding lectin (MBL), ficolin) which recognise microbial glycans (they don’t recognise vertebrae glycans)
MBL protects against N. meningitidis infection, and Ficolin-L protects against S. pneumoniae infection
MASP-1 and MASP-2 are the MBL asociated serine proteases
Briefly describe the classical pathway?
It’s similar to the lectin pathway except it uses the C1 complex
C1q binds directly to: Bacterial cell wall, CRP, and complement-fixing antibodies bound to antigen (bacteria)
C1r and C1s are related to MASP-2, they are enzymatic components that cleave complement
On binding to a pathogen surface, what occurs in order for classical C3 convertase to be formed?
On binding to a pathogen surface, MASP-2 (lectin pathway) or C1S (classical pathway) is activated. They cleave C4, and then C2 to form classical C3 convertase (C4bC2a)
C3 convertase then converts C3 to C3a (released into extracellular fluid) and C3b (binds to surface)

After C3b is bound to the microbial surface, how does the alternative pathway proceed?
- C3b deposited by classical or lectin pathway C3 convertase
- C3b binds factor B, which is then cleaved
- C3bBb complex is formed, which is the alternative pathway C3 convertase, which is then stabilised by Factor P (Properdin deficiency associated with N. meningitidis infection) - it is also destabilised by Factor H

What is the main result of the formation of C3 convertase?
They deposit large numbers of covalently bound C3b fragments on pathogen surfaces
The C3b is recognised by phagocytes, and that pathogen becomes engulfed and destroyed
What effect does the release of C3a and C5a have?
They promote inflammation and recruit more phagocytic cells to the site of infection, and they help build the MAC
How is C5 convertase generated, and what is produced as a result?
By the binding of C3b to C3 convertase (C3bBb for alt. pathway, C4b2a for classicla and lectin pathway) to form C5 convertase (C3b2Bb for alt. pathway, C4b2a3b for classical & lectin pathway)
C5 binds to C5 convertase, and is cleaved into C5a and C5b
C5a is then released (anaphylotoxin), whereas C5b binds to pathogen surface and initiates the formation of MAC

What does the formation of the MAC result in?
The MAC destroys membrane integrity, resulting in a loss of the proton gradient, which eventually kills the pathogen
What does the release of C5a and C3a result in?
C5a and C3a activates endothelium, where they cause vasodilation, an increase in vascular permeability and they increase the expression of cell adhesion molecules
They also cause mast cells to release histamine and TNFa
The increased vascular permeability increases fluid leakage into the ISF, causing the extravasation of Ig and complement
C5a activates neutrophils and monocytes, which increases their expression of cell-adhesion molceules, causes chemotaxis which results in the migration of monocytes and neutrophils, which results in increased phagocytosis by macrophages and neutrophils

When Strep. Pn binds to a receptor and crosses the blood brain barrier into the CSF, why is it able to replicate more easily than in the blood?
There is not much complement in the CSF relative to the blood stream, and there are only a few resident macrophages which are able to recognise the Strep pn. by their PAMPs (peptidogylcans) with their TLR. When strep pn is recognised, there is a release of cytokines which causes a recruitment of neutrophils and this leads to inflammation
How are neutrophils recruited into the CSF following the release of cytokines by resident macrophages?
The cytokines and chemokines cause the endothelium to express adhesin receptors that allow for the recruitent of neutrophils into the subarachnoid space, and then they follow the gradient of chemokines to the pathogen

How do neutrophils cross the blood brian barrier to get into the subarachnoid space?
They do rolling adhesion and then tight adhesion
During rolling adhesion the Sialyl-Lewis receptors on neutrophils bind to E-selectin on endothelium, where they have a Lectin-glycan interaction, this slows them down
Once the neutrophils slow down they undergo tight adhesion. During tight adhesion following rolling adhesion, LFA-1 receptors on neutrophils bind to the endothelial ICAM-1 receptors, where they have an integrin-integrin interaction

Why are the host defense mechanisms in the subarachnoid space inadequate to control infection?
There is minimal complement in CSF, which is required for efficient phagocytosis in absence of antibody
And because the IgG concentrations are low in normal CSF (increased in meningitis but still low when comapred with serum)
What effect does the capsule of S. pneumoniae, N. meningitidis, and H. influenzae have on the immune response?
The capsule masks complement and antibody, which inhibits opsonisation and phagocytosis
If order for antibodies to opsonise these capsulated bacteria, they need to be an anti-capsular antibody, which bind to the capsule and allow for efficient opsonisation and phagocytosis
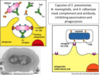
How are non-opsonised bacteria cleared from the blood?
The spleen is the main organ that clears poorly and non-opsonised bacteria from the blood (liver important for clearance of opsonised bacteria)
Splenectomized patients have an increased risk of overwhelming infections
How are poor and non-opsonised bacteria removed from the blood in the spleen?
Macrophages detect and remove bacteria from slow moving blood passing through spleen. The slow speed causes prolonged contact time between the blood and macrophages.
Which cells lie in the marginal zone in the spleen? And what do they produce
Marginal zone B cells produce IgM to polysaccharide capsule (polysaccharide is a thymus - independant antigen)
These cells are rare at birth and accumulate with age
Why should infants not be given polysaccharide vaccines?
They have very few marginal B cells in the spleen, so they will be unable to produce sufficient thymus-independant polysaccharide IgM
We must give them conjugate vaccines - however with conjugate vaccines we must give them booster shots to maintain sufficient antibody concentration to remain protective


