Organic Chemistry Flashcards
(141 cards)
What is the IUPAC name of the following molecule?
2,3-dimethylpentane
1-t-butyl-2-methylbutane
2,3-dimethylhexane
2,2,3-trimethylpentane

2,2,3-trimethylpentane
What is the IUPAC name of the following molecule?
- 4-ethyl-5-methylhexane
- 3-isopropylhexane
- 3-ethyl-2-methylhexane
- 3-isopropylcyclohexane

3-ethyl-2-methylhexane
What is the IUPAC name of the following molecule?
- 2-hydroxy-2,7,7-trimethyloctane
- 2,7-trimethyl-7-octanol
- 2,7,7-trimethyl-2-octanol
- 2,7-trimethyl-2-octanol

2,7,7-trimethyl-2-octanol
What is the name of the following molecule?
3-ethyl-2,2,4,5-tetramethylheptane
3-tert-butyl-4,5-dimethylheptane
3,4-dimethyl-5-tert-butylheptane
3,5-diethyl-2,2,4-trimethylhexane

3-ethyl-2,2,4,5-tetramethylheptane
What is the IUPAC name of the following molecule?
7-ethoxy-2,3-dimethyloctane
2-ethoxy-6,7-dimethyloctane
2,3-dimethyl-7-ethoxyoctane
2-ethoxy-6-isopropylheptane

7-ethoxy-2,3-dimethyloctane
What is the name of the following molecule?
2-propyl-4-methylhexane
4,6-dimethyloctane
3,5-dimethyloctane
4,6-diethyloctane

3,5-dimethyloctane
What is the IUPAC name of the following molecule?
4-methyl-2-vinylpentane
2-methyl-3-pentyne
4-methyl-2-pentyne
4-methyl-3-pentyne

4-methyl-2-pentyne
What is the name of the following molecule?
phenylpropanoate
phenylethanoate
ethoxybenzenone
ethyl benzoate

ethyl benzoate
What is the IUPAC name of the following molecule?
1-butanenitrile
pentanenitrile
1-cyanobutane
1-butanamine

pentanenitrile
What is the IUPAC name of the following molecule?
3-ethyl-2-methylhexane
4-ethyl-5-methylhexane
3-isopropylhexane
3-isopropylcyclohexane

3-ethyl-2-methylhexane
What is the IUPAC name of the following molecule?
4-ethyl-5,7-dimethylnonane
5,7-dimethyl-4-ethylnonane
6-ethyl-3,5-dimethylnonane
2-ethyl-4-methyl-5-propylheptane

6-ethyl-3,5-dimethylnonane
What is the name of the following molecule?
2-methoxycyclopentanone
2-methoxy-1-pentanone
methylcylcopentanoate
methoxycylcopentanal

2-methoxycyclopentanone
What is the IUPAC name of the following molecule?
1,1-dimethyloctanamide
N,N-dimethylnonanamide
N,N-dimethylnonanamine
1,1-dimethylnonanamine

N,N-dimethylnonanamide
What is the IUPAC name of the following molecule?
cyclopentanamide
cyclopentanamine
1-aminopentane
1-aminocyclopentane

cyclopentanamine
What is the IUPAC name of the following molecule?
4-ethoxy-2-methylbutane
6-methyl-3-hexanone
1-ethoxy-3-methylbutane
ethyl isopentyl ether

1-ethoxy-3-methylbutane
What is the IUPAC name of the following molecule?
1-oxopentane
pentaldehyde
1-pentanone
pentanal

pentanal
What is the IUPAC name of the following molecule?
5-cycolpentylpentanoic acid
4-cyclopentylbutanoic acid
4-carboxybutylcycolpentane
carboxypropylcyclopentane

4-cyclopentylbutanoic acid
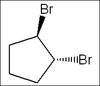
What is the name the following molecule?
1-methyl-2-ethyl-4-bromocyclohexane
1-bromo-3-ethyl-4-methylcyclohexane
5-bromo-2-ethyl-1-methylcyclohexane
4-bromo-1-ethyl-2-methylcyclohexane

4-bromo-1-ethyl-2-methylcyclohexane
What is the IUPAC name of the following molecule?
2,3-dimethyl-5-chlorohexane
4,5-dimethyl-2-chlorohexane
5-chloro-2,3-dimethylhexane
2-chloro-4,5-dimethylhexane

5-chloro-2,3-dimethylhexane
What is the IUPAC name of the following molecule?
7-hydroxy-3-heptanone
heptan-3-on-7-ol
3-keto-7-heptanol
5-oxo-1-heptanol

7-hydroxy-3-heptanone
What are the hybridization and bond angles respectively around the indicated atom?

sp2, 120
The indicated atom has 3 electron domains. According to VSEPR theory, the bond angles will be maximized and be ~120 . For 3 electron domains, the atom is sp2hybridized. Ultimately, with 3 electron domains it will need 3 hybrid orbitals and will need to mix 3 orbitals to make 3 hybrids. The 1st 3 orbitals available for making hybrids are an ‘s’ orbital and 2 ‘p’ orbitals, hence sp2.
How many sigma bonds are in the following molecule?

To be careful, you should draw out all of the bonds to the hydrogens that aren’t shown in the original structure.* There are 24 single bonds, 2 double bonds and 1 triple bond.* All single bonds are sigma bonds and the 1st bond of a double or triple bond is always a sigma bond.* The gives us 24+2+1=27

How many pi bonds are in the following molecule?

4
What are the hybridization and approximate bond angles respectively around the indicated atom?

sp3, ~109.5
The indicated atom has 4 electron domains.
According to VSEPR theory, the bond angles will be maximized and be ~109.5 (technically they’ll be a little less than 109.5 due to the repulsion of the non-bonding pair of electrons).
For 4 electron domains, the atom is sp3hybridized. Ultimately, with 4 electron domains it will need 4 hybrid orbitals and will need to mix 4 orbitals to make 4 hybrids. The 1st 4 orbitals available for making hybrids are an ‘s’ orbital and 3 ‘p’ orbitals, hence sp3.