Pediatrics Flashcards
(128 cards)
How do you caculate PGA?
Post-gestational age
weeks gestation @ birth +current age in weeks
What is a neonate? Preterm?
Low birth weight?
neonate= birht - 30 days
preterm <37 weeks
low birth weigth <2500 grams
What is extremely low gestational age (ELGAN)
23-27 weeks gestations; all organs immature
most vulnerable peds patient
What are pre-terms at risk for?
- Respiratory distress
- apnea
- hypoglycemia
- electolyte disturbance (particularly hypomagnesemia and hypocalcemia)
- infection
- hyperbilirubinemia
- polycythemia
- thrombocytopenia
Definitions for neonatal period?
Normal gestation? Postmature?
Risks for both age groups?
- Normal gestation 37-42 weeks
- all gestational ages have risk for
- congenital abnormalities,
- viral infection,
- perinatal depression,
- fetal alcohol syndrome
- thrombocytopenia
- all gestational ages have risk for
- Postmature >42 weeks
- risk of meconium aspiration
- birht trauma if large for gestational age (LGA)
- hypoglycemia
- hyperbilirubinemia
- plus above risks for normal gestations.
What is the significance of 60 weeks PGA?
What should you always evaluate?
- Former premature infancts up to 60 weeks PGA are at increased risk for postoperative apnea and braydcardia
- requires postop monitoring, admission, and 12 hour period free of apnea
- Always evaluate perinatal history
- gestation age and size at birth
- maternal infections
- eclampsia
- diabetes
- drug abuse
- prolonged or traumatic labor
- NICU/Intubation following delivery
Why is size at birth important?
- small or large for gestational age babies are more likely to have problems with metabolic, developmental, infectious or structural abnormalities, drug addiction and withdrawl
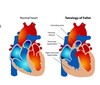
Characteristics of fetal circulation?
- High pulmonary vascular resistance and low systemic circulatory resistance
- minimal intrauterine pulmonary blood flow: only 10% CO
- At birth, placenta is no longer primary source for oxygenated blood
- Basics
- placenta–> umbilical vein–> liver sinusoids and ductus venosus–> IVC–> RA–> foramen ovale (because of pressure, blood shoots across here)–> LA (small amt of mixing with blood from pulmonary veins)–> LV–> ascending aora–> heart, brain UE (most oxygenated blood)–> mixing with deoxygenated blood from ductus arteriosus–> mixed blood feeds thoracic/abd brances–> end of aorta gives 2 umbilical arteries that return blood to placenta
- Blood from SVC mixes with blood in RA–> RV–> 10% goes pulmonary artery to lungs, most blood goes–> ductus arteriosus–> aorta arch that mixes with pre-ductal blood

What are some primary changes that occur in the transition from fetal circulation to birth?
- Ductus venosus closes
- blood is oxygenated via lungs
- ductus arteriosus closes (due to increased arterial O2 concentration)
- pulmonary vascular resistance DECREASES
- peripheral vascular resistance increases
- foramen ovale closes
- true closure weeks later; 25-30% of adults have patent foramen ovale
- only a functional closure at birth
- all these changes can reverse in stressed newborn.

What is transitional ciruclation?
Prevention? Treatmenbt?
- occurs at birth for the first several weeks
- hypoxia, hypercapnia, or hypothermia can lead to increased pulmonary artery pressure, which causes reversal of flow through foramen ovale (meaning returning to fetal circulation), reopening of ductus arteriosus and shunting
- deoxygenated blood perfuses systemic circulation and this hypoxia is difficult to correct
- prevention: optimal oxygenation, correct acidosis, keep warm
- treatment: hyperventilate to reduce PaCO2
Characteristics of newborn heart?
- Newborn heart:
- structurally immature
- fewer myofibirls (not parallel)
- sarcoplasmic reticulum immature and cardiac calcium stores reduced
- ventricles less compliant: CO is HR dependent
- baroreceptor reflex immature, won’t have increase in HR in response to decrease BP
- Heart not as responsive to volume c/t adult
- PSNS dominance- immature SNS, and much more likely to have bradycardia with any kind of stress/suctioning/ etc
- premedicate prior to DL/suction etc
Resting cardiac ouput for neonate, infant, and adolescent?
- Neonate at birht 400mL/kg/min
- Infant 200 mg/kg/min
- Adolescent 100 mL/kg/min
CV characteristics in neonate?
- Dependence on ionized calcium- particularly vulnerable to effects of citrated blood products
- also vulnerable to myocardial depression caused by potent anesthetics
- Neonate myocardium relatively noncompliant c/t older kids
- increased preload does not increase SV to same degree
- poor tolerance to increase afterload (development of BiV failure)
- hypovolemia and bradycardia produce dramatic decrease in CO that threaten organ perfusion
- Epinephrine rather than atropine increases contractility and HR
- preferred txmt of bradycardia and decreased CO in ped patients
- In 1st 3 months, heart does not respond as well to inotropic support
- immature beta respons
*
- immature beta respons
Pulmonary system in infants?
- Alveoli increase in number & size up until 8yo
- Infants: small airway diameter; increased resistance
-
Highly compliant airway & chest wall
- however, lung tissue not as compliant. less elastin tissue. this can lead to airway collapse and chest wall collapse
- Closing capacity is greater than FRC in the very young and very old: airway closure can occur before end exhalation
- Early fatigue of diaphragmatic & intercostal muscles until age 2 (type 1 muscle fibers not mature)
- only 10% type 1 muscle fibers in the diaphragm in infant. adult has 55%
-
Highly compliant airway & chest wall
-
O2 consumption is 2-3 x’s the adult with increased alveolar ventilation; leads to rapid desaturations especially during cold stress and in the case of airway obstruction
- MV: FRC ratio 2-3 x higher than adult causes faster anesthetic onset, fast desat and less O2 reserve
- Angulation of right mainstem bronchus

Airway differences in infant?
- Infant:
- larger tongue in smaller submental space
- higher larynx(C2 to C4)
- short stubby (omega shaped) epiglottis
- angled vocal cords (slant caudally)<bolded></bolded>
- funnel shaped larynx with narrowest region @ cricoid ring
- obligate nasal breathers
- large occiputs & the “sniffing” position is favored for axis alignment
- shoulder roll useful. large head c/t body, no hyperextension!
- endentulous
- short trachea (4-5 cm)
- Tooth eruption normally occurs between 4 and 12 months of age for the first tooth; eruption of the 20 primary teeth should be complete between 24 and 30 months of age.

Gas flow in young children?
- Young children have elevated airway resistance at baseline
- Turbulent airflow is present to 5th bronchial division
- A 50% reduction in radius increases the pressure 32-fold
- Very prone to respiratory distress with any upper airway irritation or swelling
- laminar flow R to 4th power (poiseuille’s law!)
- turbulent radius to 5th power–> even more reduction in flow

Neurological characteristics of infant?
O2 consumption? Growth of brain? Location of conus medullaris?
Fontanel closure (ant and post)?
- Oxygen consumption & CBF in the brain of children is ~50% greater than adults
- O2 consumption
- infant 5.5 mL/100g/min
- adult 3.5 mL/100g/min
-
CBF
- infant 100 mL/min/100g
- adult 50 mL/min/100g
- O2 consumption
- Myelinization & synaptic connections not complete until age 3-4 years
- Rapid growth of brain in first 2 years of life
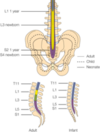
- Conus medullaris is at level of L3 at birth & migrates to level L1-L2 by age 3
- Fontanels: anterior fontanel closed by 18 mo’s; posterior fontanel closed by ~2 mo’s
What is anesthesia induced developmental neurotoxicity?
Anesthesia-Induced Developmental Neurotoxicity: our knowledge is still growing in this area
- Increased and accelerated neuroapoptosis with virtually all anesthetics
- Single exposures of short duration are usually of no consequence
-
Repeated &/or prolonged exposures at a young age (<3-4 years) may be associated with later behavioral & learning difficulties- we do not have conclusive evidence
- __current thought to delay elective/non-urgent sx until children >3-4 yrs from neurocognitive standpoint. have not proven delays
-
most GA cause morphology changes in developing brain
-
some human sutdies have gound association b/w exposure to aneshesia and surgery in early childhood
- may be explained by confounding factors
- increasing evidence shows one hour of aneshteisa in infancy does not have lasting impact on cognition
-
some human sutdies have gound association b/w exposure to aneshesia and surgery in early childhood
Neuraxial considerations in pediatrics
- The conus medullaris ends at approximately L1 in adults and at the L2–L3 level in neonates and infants.
- In infants, the line across the top of both iliac crests (the intercristal line) crosses the vertebral column at the L4–L5 or L5–S1 interspace, well below the termination of the spinal cord
- The dural sac in neonates and infants also terminates in a more caudad location compared to adults, usually at about the level of S3 compared to the adult level of S1
- Infants: lack of a lumbar lordosis compared to older children predisposes the infant to high spinal blockade with changes in positioning
Renal characteristics of infants?
-
GFR is significantly impaired at birth but improves throughout the 1st year
- greatest impairment is in 1st 4 weeks of life
- renal maturation will be delayed further with prematurity
- UOP low at birth x 24 hours then increases to 1-2mL/kg/hr
- be concerned after 24 hours with low UOP
- in utero kidney only receives 3% blood flow. adult 25%.
- Renal tubular concentrating abilities do not achieve full capacity until ~2years
- difficulty with concentrating and diluting urine
-
does not respond as well to aldosterone
- hypo/hypernatremia can easily become an issue
- Half-life of medications excreted by glomerular filtration are prolonged in the very young (antibiotics; etc.)
- In contrast, during childhood, renal clearance rate may increase to levels higher than even adult clearance rates
- higher CO, more blood flow in childhood
Liver function in infants?
- Enzyme systems are still developing up until 1 year of age
- Phase I Cytochrome P450 system is 50% of adult values at birth
- 3A4 50% drugs
- 2D6= 10-20% drugs
- Phase II (conjugation reactions) are impaired in neonates
- Long half life of BZD and morphine
- Decreased bilirubin breakdown due to reduction in glucuronyl tranferase (leading to jaundice)- also metabolize tylenol
- Hepatic synthesis of clotting factors reach adult levels within a week of birth
-
Vit K dependent factors (II, VII, IX, X)
- at birth 20-60% adult values
- preterm values even less
-
Vit K dependent factors (II, VII, IX, X)
-
Lower levels of albumin/ other proteins for drug binding in newborns- larger proportion of unbound drug circulating
- increases effect of highly protein bound drugs.
- Minimal glycogen stores- prone to hypoglycemia
GI system in pediatrics?
- Obligate nose breathers
- Coordination of swallowing with respiration not mature until 4-5 months of age (grow out of it eventually)
- high incidence of reflux especially in pre-terms
- coanal atresia- blockage of nasal to trachea
- resp depression bc want to breathe through nose! Will breath better when crying
- coanal atresia- blockage of nasal to trachea
-
Gastric juices are less acidic (more neutral) up to ~3 years of age
- Less absorption of drugs
-
Absorption of oral medications is generally slower compared to adults (less effective)
- The gastrointestinal tract is generally slower in children than in adults
- Children have differences in gastric pH, emptying time, intestinal transit, immaturity of secretions, and activity of both bile and pancreatic fluids
What are factors that lead to difficulty in thermoregulation in infants?
- Large surface area to body weight
- Lack of subcutaneous tissue as an insulator
-
< 3 mo →Inability to shiver:metabolize brown fat to increase heat production
- can lead to metabolic acidosis & increased O2 consumption
- Brown fat: tissues in neck, vertebral column, around adrenal glands → Metabolically stressful!
- can lead to metabolic acidosis & increased O2 consumption
-
Factors: cold OR, anesthetic-induced vasodilation, room-temp IV fluids, evaporative heat loss from surgical site, cool irrigating solutions on field, cool/dry anesthetic gases
- In picture
-
Conduction- cold fluid, cold OR table (cold surface/fluid absorbing heat)**
- decrease heat loss by placing baby on warming mattress and warm surgical unit
-
Evaporation- cold gas vent to pt, cold irrigating fluid r/t heat loss
- humidifaciton of inspired gases, use plastic wrap and warm skin disinfectant solutions
-
Convection- air flowing over
- keep infant in incubator and cover with blankets
- head should be covered
-
radiation from image- giving off heat
- use double shelled isolette during transport
- % of heat loss in children: 39% radiation; 34% convection; 24% evaporation; 3% conduction

How can we maintain temperature in infants?
-
Active warming is critical:
- warm the OR (dec convection) 72-76o (or 80’s)
- as warm as everyone can tolerate in infants
- use a warming mattress
- use incubators
- cover with blankets- dec radiation
- head coverings (up to 60% of heat loss)
- transport in isolette
- humidify gases- dec evaporation
- single limb circuit**- gases getting warmed up by exhaled air
- use plastic wrap on the skin
- warm prep & irrigation solutions
- change wet diapers & remove wet clothing
- Forced air warmers: the most effective strategy to minimize heat loss in surgery in children > 1 hr
- Careful w/ injury!
- warm the OR (dec convection) 72-76o (or 80’s)
- Anesthetics alter non-shivering thermogenesis in neonates