PL rst: Modern analytical techniques: high-resolution mass spectrometry; NMR spectroscopy Flashcards
(21 cards)
High-resolution mass spectrometry measures the molecular mass of ions to four decimal places.
State one use of this technique which is not shared with regular mass spectrometry.
Differentiating between 2 molecules with the same Mr.
C2H4, N2 and CO all have a Mr of 28. Substance X has an accurate Mr of 27.9949.
Identify substance X. The exact relative atomic masses of the relevant elements are given below.


Complete the table summarising mass spectrometry and IR spectroscopy.


The environment of a carbon atom refers to the atoms or groups it is bonded to.
How many carbon environments are there in propane?
2
Central C is in one environment, and the 2 outer Cs are in the same environment since propane is symmetrical
- Draw methylpropan-1-ol.
- Annotate the diagram to show the number of carbon environments it has, and how many carbon atoms are present in each.

Read the following explanation of how 13C NMR (nuclear magnetic resonance) spectroscopy works.
- Carbon-13 nuclei behave like magnets, so can align with or oppose a magnetic field.
- Supplying the correct frequency causes them to flip from the stabler aligned state to the less stable opposed state.
- Chemical shift is related to the frequency required, relative to a standard (in this case, TMS). Lower shift indicates a higher frequency.
- The environment a 13C nucleus is in affects its shift. If a nucleus is bonded to or near to a shifting group (involving electronegative atoms), it experiences more shifting.
Use this information to explain why a 13C nucleus bonded to a shifting group experiences more shifting.

- 13C nucleus is bonded to an electronegative atom
- Bonding electrons are further from nucleus
- Nucleus experiences less shielding
- Lower frequency is required to flip it (to oppose magnetic field)
- Higher chemical shift
Carbon-13 NMR (nuclear magnetic resonance) works on the principle that carbon-13 nuclei are found in different chemical environments.
Describe what can or cannot be deduced from the following features of a 13C NMR spectrum (example below):
- % intensity (y-axis)
- Chemical shift, δ (x-axis)
- Number of peaks
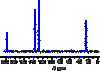
% intensity: no information (doesn’t indicate relative number of carbons in each environment)
Chemical shift: type of 13C environment
Number of peaks: number of 13C environments
An isomer of methyl propanoate has the following 13C NMR spectrum.
Suggest the structural formula for the compound, explaining your reasoning.

Molecular formula is C4H8O2
4 peaks so 4 carbon environments
There are 4 carbons so each is in a unique environment; therefore asymmetrical
- Peak around 25 ppm indicates C-C
- Peak around 60 ppm indicates C-O
- Peak around 80 ppm indicates another C-O, but more shifted
- Peak around 205 indicates C=O
There are 2 C-O environments, one being shifted by C=O. Therefore structure is HCOCH2OCH2CH3

The 13C NMR of a compound with molecular formula C3H6O is shown below.
Suggest the structure of the compound.

Possible structures: propanone (CH3C=OCH3), 1-hydroxyprop-1-ene (CH2=CHCH2OH), propanal (CH3CH2CHO).
3 13C environments. Compound contains 3 Cs so is asymmetrical.
- ~10 → C-C
- ~40 → C-C, shifted
- ~200 → C=O
Must be aldehyde or ketone: either propanone or propanal.
Not propanone since it only has 2 13C environments.
So must be propanal, CH3CH2CHO.
How many peaks would you expect in the 13C NMR spectra of the following?
- Ethane
- Propane
- Propan-1-ol
- Propan-2-ol
- 1
- 2
- 3
- 2
How many proton environments are there in this molecule?

3
check - but think the 3 methyl groups are same env due to free rotation
Proton NMR (nuclear magnetic resonance) works on the principle that 1H nuclei are found in different environments.
Describe what can or cannot be deduced from the following features of a proton NMR spectrum:
- % intensity (y-axis)
- Chemical shift, δ (x-axis)
- Number of peaks
- Area under a given peak, if labelled
% intensity: no information
Chemical shift: type of proton environment
Number of peaks: number of proton environments
Area under a peak: relative abundance of protons in the environment
How many peaks would you expect in the 1H NMR spectra of the following?
- Ethane
- Propane
- Propan-1-ol
- Propan-2-ol
- 1
- 2
- 4
- 3
Determine which of the following is responsible for the low-resolution 1H NMR spectrum below:
- Propanoic acid
- Methyl ethanoate
- Ethyl methanoate

3 peaks so 3 hydrogen environments.
- Peak around 1 ppm indicates 3 hydrogens in HC-R environment
- Peak around 2.5 ppm indicates 2 hydrogens in HC-C=O environment
- Peak around 12 ppm indicates 1 hydrogen in COOH environment
(1) could apply to any of the three.
(2) and (3) only apply to propanoic acid, so compound is propanoic acid.
In high resolution 1H NMR spectra, splitting patterns may be observed, as shown below.
What can be deduced from the number of sub-peaks in a given cluster?

n + 1 rule
Number of sub-peaks = n + 1, where n = number of hydrogens bonded to adjacent carbon(s) (to carbon to which proton(s) in question is/are bonded).
This rule derives from the fact that n protons bonded to one carbon have n + 1 possible magnetic orientations. These protons affect the chemical shift of those bonded to the adjacent carbon, and can do so in n + 1 different ways, giving rise to n + 1 sub-peaks.
State and explain how many 1H NMR sub-peaks which a proton in a CH3-OH environment would cause.
- One (singlet).
- Proton is not bonded to a carbon, so n + 1 rule cannot apply (electronegative atoms prevent splitting)
State and explain whether there is splitting in the proton NMR spectrum of ethane.
No, since hydrogens on each carbon are in identical environments.
You don’t get splitting if hydrogens are in same environment, because they resonate (flip magnetically) at same time
Suggest the name of the compound which produces this proton NMR spectrum.

2 peaks, so 2 proton environments, so must have 2 carbons, or 3 with symmetricality.
- Triplet around 0.9 ppm indicates hydrogens(s) in HC-R environment, with adjacent carbon(s) bonded to 2 hydrogens
- Heptet (7 sub-peaks) around 1.3 ppm indicates hydrogens(s) in HC-R environment, with adjacent carbon(s) bonded to 6 hydrogens
Must be alkane (due to environments).
Heptet must indicate 2 adjacent carbons each bonded to 3 hydrogens, all in same environment.
So must be propane, CH3CH2CH3.
The 1H NMR spectrum of an ester is shown below. Work out the structure of the ester.

3 peaks, so 3 hydrogen environments, in ratio 1:3:6.
- Doublet ~1 ppm → 6 Hs in HC-R environment, with adjacent carbon attached to one hydrogen. So (CH3)2-CH
- Singlet ~2 ppm → 3 Hs in HC-C=O environment, with no Hs on adjacent carbon. So H3C-OC-
- Multiplet ~5 ppm → 1 hydrogen, with adjacent carbon attached to several hydrogens. Cannot be C=CH environment, since this doesn’t fit 1:3:6 ratio, so must be HC-R, but shifted by an electronegative atom
Structure must be CH3COOCH(CH3)2
A compound with molecular formula C4H8O2 produces the 1H NMR spectrum below.
Work out the structure of the compound.

3 peaks, so 3 hydrogen environments, in ratio 2:3:3.
- Triplet around 1 ppm indicates 3 protons in HC-R environment, where adjacent carbon is bonded to 2 hydrogens: so H3C-CH
- Singlet around 2 ppm indicates 3 protons in HC-C=O environment, with no hydrogens on adjacent carbon (or no adjacent carbon): so H3C-C=O
- Quartet around 4 ppm indicates 2 hydrogens in HC-O environment, where adjacent carbon is bonded to 3 hydrogens: so H3C-CH2-O
So structure must be CH3COOCH3CH3

Complete the table summarising NMR spectroscopy.




