Review Flashcards
(33 cards)
At what difference in electronegativity does an ionic bond form between two atoms?
> 2.0
At what difference in electronegativity does a polar bond form between two atoms?
> 0.5
What is the formula for formal charge?
formal charge = (# of valence e-) - (# of e- atom “owns”)
What is the formula to find out how many e- an atom “owns”?
of e- atom “owns” = (# of unpaired e-) + (1/2)(shared)
Stability rules
- Fulfills octet rule 2. More bonds is more stable 3. Minimized charge 4. More stable if negative charge is on most electronegative ion
How does pka relate to the strength of the acid and the Ka value?
lower pka = stronger acid = higher Ka
pka of alkane
~50
pka of amine
~35
pka of alkyne
25
pka of water
16
pka of protonated amines
10
pka of carboxylic acids
5
pka of hydrochloric acid
-7
What four variables affect the stability of the conjugate base?
- element - inductive (electron withdrawing) - resonance - hybridization
Lewis base
donates an e- pair; nucleophile
Lewis acid
accepts an e- pair; electrophile
What are heteroatoms?
any atoms that are not carbon or hydrogen
aliphatic: alkanes
have a single bond between carbons
aliphatic: alkenes
have a double bond between carbons
aliphatic: alkynes
have a triple bond between carbons
aromatic compounds
rings

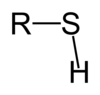
Alkyl Halide
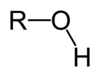
Alcohol (hydroxyl)

Ether (alkoxy)