sensory processing and pain Flashcards
(33 cards)
where does sensory innervation to the head come from?
from the trigeminal ganglion that is adjacent to the pons
describe anatomy of the peripheral sensory nervous system
draw and label

where are pain sensing neurons found?
in the dorsal root ganglion
what are the three major sub-classes of adult DRG neurons?
what is their % of DRG neurons?
- proprioceptive neurons
- nociceptive neurons (60-65%)
- low threshold mechano-receptive (LTM) neurons (25-30%)
describe proprioceptive neurons
- large-diameter soma
- large calibre, thickly myelinated axons
- their central projections terminate in intermediate zone and ventral horn of the spinal cord
- they innervate skeletal muscle spindles & golgi tendon organs to provide spatial awareness of the limbs

describe LTM neurons
low threshold mechano-receptive neurons - 25-30% of DRG neurons
- large to medium diameter cell bodies
- thickly myelinated axons
- their central projections branch and project up dorsal columns and go to deep dorsal horn
- innervate specialised receptor organs in skin dermis, epidermis and deeper tissue layers
- detect light touch, gentle tissue deformation, allow discrimination between different textures etc.
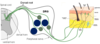
describe nociceptive neurons
60-65% of DRG neurons
- small to medium diameter cell bodies
- small calibre axons
- thinly myelinated or non-myelinated
- their central projections terminate in superficial dorsal horn
- most peripheral fibres end as naked terminal arbors in skin or viscera
- transmit information on painful stimuli, physiological temperatures and itch.
where are nociceptive neuron fibre tracts carrying nociceptive information found?
found in the anterolateral region of the spinal cord
what are the pathways of ascending fibre tracts carrying nociceptive information called?
how are they classified?
Direct anterolateral pathway:
-
spinothamalamic tract
- From spinal cord to thalamus
Indirect anterolateral pathways:
-
spinoreticular tract
- To reticular formation in hind brain
-
spinomesencephalic tract
- To midbrain
describe the spinothalamic tract
Dorsal horn projection neurons
- Projection neurons in lamina I are nociceptive specific
- Projection neurons in lamina III to VI are WDR (noxious and non-noxious stimuli)
- Wide dynamic range neurons
Long axons that cross midline and travel up to thalamus
- Thalamus is somatotopically mapped
- Pain is localised
sharp, precisely localised pain

describ the spinoreticular tract
aka paleospinothalamic tract
- Makes connections with areas of hindbrain
- Long axon crosses midline
- Synaptic connection with nuclei of reticular formation
- Project to thalamus
- Not somatotopically mapped
- Pain is poorly localised
- Neurons project to PSC - pain is rationalised
- Perception of pain
- C-fibre nociceptors input to the spinoreticular tract predominantly via excitatory lamina II interneurons
dull, persistent, poorly localised pain and arousal response to nociceptive input

describe the spinomesencephalic tract
- Long axon passes midline
- Synaptic connection with neurons in parabrachial nucleus
- Remember in pic
- PAG
- Synaptic connection with neurons in parabrachial nucleus
- C-fibre nociceptors input to dorsal horn projection neurons via excitatory lamina II interneurons

ANS response to painful stimuli
emotional response to painful stimuli
input to centres controlling descending modulation of pain
what are the benefits of pain?
protective mechanism to minimise tissue damage

what can prolonged hyperalgesia lead to?
what is it caused by?
- results from central sensitisation of second order interneuron and/or peripheral sensitisation of primary nociceptor
- results in debilitating persistant pain
- osteoarthritis
- interstitial cystitis
- pancreatitis
describe the spinal pain reflex
draw diagram and label
removal from source of danger
- innervated by a heat pain
* responsive nociceptive neuron - Excitatory interneuron projects axon to motor neuron in ventral horn
* green - Motor neuron innervates hand and arm muscle
* Minimise damage to skin
- A-delta myelinated fibre is the nociceptor involved
- Faster than c-fibre
- Nociceptor branches in the dorsal horn to also synapse with a projection interneuron that carries the pain signal to the brain
- Orange

how does temperature perception occur?
Molecules allow us to perceive temperature
Physiological and noxious temperatures are detected by ion channels in temperature responsive and nociceptive neurons
These ion channels change conformation and open when the temperature changes allowing Na+ and Ca2+ to pass through them
- -> membrane depolarisation
Individual neurons only express one type of temperature responsive ion channel
what are the hot detecting ion channels?
TrpV1 can also be activated by capsaicin (active ingredient in chilli)

what are the cold detecting ion channels?
Expressed by dorsal root ganglion neurons
TrpM8 can also be activated by menthol

how does tissue damage activate nociceptiors?
Tissue damage produces chemical that activate and sensitise nociceptors
- skin damage
- detected by nociceptive DRG neuron - as it terminates in superficial layers of dorsal horn
- skin cut
- dying cells release K+ ATP and H ions
- K+ ions directly depolarise nociceptor terminals
- ATP binds to P2X3 ion channels - opens & causes depolarisation
- H+ activates ASICS - depolarises nociceptors
- tissue damage also releases enzymes that contribute to hyperalgesia
- cyclo-oxygenase enzymes
- make PGE2 - increase sensitivity of nociceptors
- bradykinin
- binds to GPCR - depolarises
- sensitises TrpV channels to open at lower temperature
NSAIDS reduce pain by inhibiting cox

what is substance P and what its function?
- Released by depolarised nociceptors
- Can bind to receptors on capillaries
- Makes leaky
- Local swelling
- Binds to receptors on mast cells
- Causes degranulate
- Empty contents into skin
- Histamine
- Binds
- Makes capillaries leaky - swelling
- PGE
- ATP
- Bradykinin
- Histamine
- Empty contents into skin
- Causes degranulate
- Sensitises nociceptors to be activates by lower intensities of noxious stimuli

what is the role of nerve growth factor (NGF)?
- Major role in inducing chronic hyperalgesia
- Normally produced in skin
- Role in maintaining function and health of nociceptor and sensory neurons
- If tissue is damaged
- Level of NGF in target fields of sensory neurons rapidly increases
what are the early effects of NGF in peripheral nociceptor sensitisation?
- NGF is rapidly up-regulated in target fields of nociceptive neurons after tissue trauma
- NGF rapidly phosphorylates the heat channel TrpV1 lowering its threshold for activation
- Activates at body temperature - painful
- NGF facilitates mast cell degranulation
what are the late effects of NGF in peripheral nociceptor sensitisation?
- NGF increases the expression of many peptides and ion channels in nociceptive neurons including
- Substance P
- Heat responsive channels
- TrpV1
- TrpA1
- TrpM8
- Acid sensing ion channels (ASICS)
- TTX-resistant voltage-gated sodium channels
- Nav1.8
- Nav1.9
= enhanced sensitivity of nociceptive neurons to noxious stimuli
what are the effects of NGF in central sensitisation (sensitisation of the second order interneuron)?
- NGF up-regulated substance P is released at the central terminals of nociceptive neurons (dorsal horn)
- NGF promotes the synthesis of brain derived neurotrophic factor (BDNF) in nociceptive neurons and promotes BDNF release from their terminals in the dorsal horn
->
- Centrally released SubP and BDNF promote increased excitability of second order interneurons that project to the thalamus in the spinothalamic tract Long term potentiation
- Increased synaptic efficiency
- = projection interneurons are more sensitive to nociceptive input from DRG neuron terminals






