Cellular Signaling
production, release, detection, & response to extracellular signaling molecules
Receptor
protein that binds to or is activated by a signaling molecule leading to initiation of cellular response
Ligand
signaling molecule that binds to a receptor
Plasma Membrane-bound Receptor
binds extracellular signaling molecule in extracellular domain or membrane spanning domain
induces conformational change to cytosolic domain initiation of cellular response
Signal Transduction
process of converting a chemical signal into a cellular response
Intracellular Receptor
cytosolic or nuclear membrane receptors for signaling molecules that pass through the plasma membrane
Reception
binding of signaling molecule to receptor
Transduction
initiation of 1 or more pathways activated by the receptor
Response
specific change in cellular function
Signaling cell
cell that produces & transmit signal
Target cell
cell that receives signal & responds
Autocrine signaling
when a cell is both the signaling & target cell
cell produces ligand for the receptors on its surface
ex. some growth factors
IMPORTANT for tumor cell growth
Paracrine signaling
target cells are in close proximity to signaling cells
ex. neurotransmitters
Neurotransmitters
small signaling molecules release from one neuron to the next or terminal target over a synapse
Endocrine signaling
target cells are distant from signaling cells usually travel in the blood
ex. hormones from thyroid, pituitary, adrenals
Hormones
cholesterol derived endocrine signaling molecules
Cell-to-cell signaling
plasma membrane-bound receptor & plasma membrane-bound ligand interaction between adjacent cells
Types of Cellular Response
- changes in activity or function of proteins
- quick (seconds to minutes)
- transcriptional regulation
- slow (minutes to hours)
Disassociation Constant
Kd = ([R][L])/[RL] = koff/kon
Kd is inversely proportional to affinity
Saturation Kinetics
@ [L] >> Kd
almost all receptors are bound by ligand
Bmax
Maxium binding
Effector Specificity
specific cellular response mediated by each specific receptor-ligand complex
many signaling molecules can bind multiple types of receptor, but the cellular response will be different
Acetylcholine
ACh; neurotransmitter
- binds & activates ligand-gated channel in straited muscles causing contraction
- binds & activates a G-protein coupled receptor that slows rate of contraction of the heart
- binds pancreatic receptors to trigger release of digestive enzymes
GPCR
G-Protein Coupled Receptor
large group of cell surface receptors that are coupled to trimeric G proteins
7 transmembrane domains, 4 each extracellular & intracellular segments, N terminal on extracellular side & C terminal on intracellular side
C3 & C4 (cytocsolic segments) interact with G protein
Sensitivity
directly dependent to the number of receptors
thus regulating # of receptors is another control mechanism
First Messenger
extracellular signaling molecule
binding to receptor usually causes short lived increase or decrease in second messsenger molecule
Second Messenger
intracellular signaling molecule
common: cAMP, cGMP, DAG (1,2-diacylglycerol), IP3 (Inositol 1,4,5-triphosphate), Ca2+, phosphoinositides
inc [2nd messenger] usually triggers rapid change in activity of 1 or more enzymes and/or non-enzyme proteins
GTPase Switch Proteins
catalyze GTP hydrolysis to GDP
signal transduction forms: OFF→bound to GDP & ON→bound to GTP
two classes: trimeric & monomeric
Trimeric G proteins
large, bind directly to & activated by receptor
alpha, beta, & gamma subunits
beta & gamma are always together & covalently linked to lipid molecule of plasma membrane
alpha subunit is GTPase switch & covalently linked to lipid molecule of plasma membrane
Monomeric G protein
small, linked indirectly to receptors via adaptors
Protein Kinase
adds PO43- to intracellular protein
usually from terminal end of ATP
2 types: tyr or ser/thr
addition of phosphate group can activate or inhibit the action of the protein
phosphorylation at different site produces a different result
many proteins are substrates for multiple kinases
some cell surface receptors have kinase activity
Protein Phosphatases
opposite of protein kinases
removal of PO43-
some cell surface receptors have phosphatase activity
removal of phosphate group can activate or inhibit the action of the protein
Signal Transduction Regulation Mechanisms
- degradation of second messenger
- desensitization of receptor
- modification to activity
- endocytosis of receptor
- deactivation of signal transduction proteins (GTPase bound signaled to hydrolyze GTP→GDP)
Effector protein
activity modulated by trimeric G protein of GPCR (usually alpha subunit, but can be beta-gamma), usually activation
either membrane-bound ion channels OR 2nd messengers
Galpha
GTPase switch protein subunit of trimeric G protein in GPCRs
four classes: s, i q, & t
GSalpha
effector protein: adenylyl cyclase
effect on 2nd messenger: inc. cAMP
ex: beta1-adrenoreceptor, beta2-adrenoreceptor, glucagon receptor
GIalpha
effector protein: adenylyl cyclase; K+ channel (activated by beta-gamma subunit)
effect on 2nd messenger: decr. cAMP; change in membrane potential
ex: alpha2-adrenoreceptor, glucagon receptor; M2 mAChR
GQalpha
effector protein: phospholipase C
effect on 2nd messenger: inc. IP3/DAG
ex: alpha1-adrenoreceptor, M3 mAChR
GTalpha
effector protein: cGMP phospohdiesterase
effect on 2nd messenger: decr. cGMP
ex: rhodopsin
Mechanism of GPCRs
- signal binding to receptor induces conformational change to bind Galpha/GDP
- displacement of GDP, so Galpha binds GTP
- Galpha/GTP dissociates from Gbeta-gamma (both remain attached to plasma membrane)
- Galpha/GTP binds & regulate effector protein
- GTP is hydrolyzed to GDP
Adenylyl cyclase
plasma membrane-bound enzyme that catalyzes: ATP→ cAMP + PPi (pyrophosphate)
2 catalytic domains & 2 integral membrane proteins
Epinephrine
response to stress/ fight or flight & heavy exercise
stimulates glycogenolysis & lipolysis
increase contraction rate of heart muscle
increase relaxation of intestinal smooth muscle
increase constriction of intestinal, skin, & renal vasculature (divert to muscle)
different effect on different GPCRs all regulate adenylyl cyclase
beta1-adrenoceptor
GPCR with GSalpha
binds & stimulates adenylyl cyclase
causes increase [cAMP]
expressed in myocardium, juxtaglomerular cells, some presynaptic terminals, adipocytes, CNS neurons
beta2-adrenoceptor
GPCR with GSalpha
binds & stimulates adenylyl cyclase
causes increase [cAMP]
expressed in visceral smooth muscle, vascular smooth muscle, liver, myocardium, skeletal muscle, some presynaptic terminals & CNS neurons
Cholera
severe diarrhea that leads to death through rapid dehydration
vibrio cholerae bacterium
produces cholera toxin protein complex in small intestine, internalized into intestinal mucosal cells, ADP-ribosylation of GSalpha (turns permenantly ON), continuous cAMP production, excessive secretion of water & electrolytes to intestinal lumen
feedback inhibition
when downstream product in a molecular pathway inhibits its own production
usually at the first irreversible step
catabolic pathway regulation
pathway used to make ATP, therefore inhibited by large amounts of ATP & stimulated by high amounts of ADP
Feedforward Stimulation
when nutrients or initial reactants stimulate their own metabolism
GLUT1
high affinity glucose carrier
found in brain, RBCs & others
GLUT2
low affinity glucose carrier with high capacity
found in liver & intestine
GLUT3
neuronal glucose carrier
GLUT4
insulin-dependent glucose carrier
found in muscle & adipose tissue
2/3 of all glucose is used this way after a meal
GLUT5
transports fructose & glucose
Glycolysis
metabolic pathway for glucose catabolism to pyruvate (10 steps)
occurs in cytoplasm
uses 2 ATP, makes 4 ATP & 2 NADH, deltaGº’= -47kcal/mol
without O2, run out of NAD+
First step of Gylcolysis
irreversible, but not comitted step because G6P is used for several pathways
requires presence of Mg2+, deltaG’º= -16.7kJ/mol

Second step of glycolysis
freely reversible, requires presence of Mg2+, deltaG’º= 1.7kJ/mol
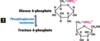
Third Step of Glysolysis
first irreversible step of glycolysis= committed step
requires ATP & presence of Mg2+, deltaG’º= -14.2kJ/mol

Fourth Step of Glycolysis
freely reversible, deltaG’º= 23.8kJ/mol
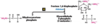
Fifth step of glycolysis
freely reversible, deltaG’º= 7.5kJ/mol

Sixth step of glycolysis
freely reversible, deltaG’º= 6.3kJ/mol
couples exergonic oxidation of aldehyde group to formation of high-energy mixed anhydride

Seventh step of glycolysis
freely reversible, deltaG’º= -4.5kJ/mol

eighth step of glycolysis
freely reversible, requires presence of Mg2+, deltaG’º= 4.4kJ/mol

ninth step of glycolysis
freely reversible, deltaG’º= 0.4kJ/mol

tenth step of glycolysis
irreversible, requires presence of Mg2+ & K+, deltaG’º= -31.4kJ/mol

arsenic poisoning
arsenic forms arsenate (AsO43+) which gets added to glyceraldehyde 3-phosphate during the 6th step of glycolysis
then during 7th step, arsenate is uncoupled by water & non ATP is formed
anaerobic respiration
no oxygen→ glycolysis only
glucose + 2 Pi + 2 ADP → 2 lactate + 2 ATP + 2 H2O
- cells without small energy requirement
- cells when O2 is limiting factor
- contracting muscle
- ischemic tissue
Toxic Thyroid Nodules
- benign thyroid adenomas from a single cell that proliferates abnormally
- sometimes arise from GSalpha mutation (no GTPase activity)→ always in active state
- overproduction of cAMP
- results in overproduction of thyroid hormones, abnormal cell proliferation, & tumor formation
alpha2-adrenoreceptor
expressed in some presynaptic terminals, pancreatic islets, platelets, ciliary epithelium, smooth muscle, & CNS neurons
binds & activates Gi-alpha (inhibitory G protein) that binds & inhibits adenylyl cyclase
decrease [cAMP]
Whooping Cough
- acute inflammation of URT causing paroxysmal coughing, inspiratory whoop, fainting &/or vomitting after cough
- bordetella pertussis secretes pertussis toxin
- toxin internalized in URT→ ADP-ribosylation of Gi-alpha (locking into off state)
- uninhibited adeylyl cyclase→ continuous cAMP production
Lactate Dehydrogenase
used to recover NAD+ in anaerobic conditions
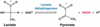
PFK-1 Regulation
Phosphofructokinase-1, allosteric enzyme
committed step of glycolysis
inhibitors: ATP, citrate, low pH, glucagon
stimulants: ADP, AMP, insulin (via Fructose 2,6-bisphosphate), beta-adrenergic agonists (released during stress & exercise, only effects muscle)
Effects of Insulin on Glycosis
- stimulates glucose uptake in muscle & adipose thru upregulation of GLUT4
- increase synthesis of glycolytic enzymes
- synthesis of allosteric effector fructose 2,6-bisphosphate
inhibits cAMP production & stimulate a protein phosphatase to remove phosphate from PFK-2 of frutose 2,6-phosphatase→ PFK-2 ON → raise levels of fructose 2,6-phosphate → actiavtion of PFK-1 → glycolysis
Effects of Glucagon on Glycosis
release during fasting
acts via enzyme synthesis & fructose 2,6-bisphosphate
activates cAMP production → stimulates Protein Kinase A → phosphorylates PFK-2 on Fructose 2,6-bisphosphatase → PFK-2 OFF → low levels fructose 2,6-bisphosphate → no actiavtion of PFK-1 → no glycolysis
Effects of Adrenaline on Glycosis
stimulates glycolysis in muscle
inhibits glycolysis in liver
Fructose 2,6-bisphosphate
potent allosteric activator of PFK-1
synthesized by PFK-2 domain of Fructose 2,6-bis phosphatase
phosphorylated PFK-2 is OFF → low levels of fructose 2,6-bisphosphate → no activation of PFK-1 → no glycolysis
unphosphorylated PFK-2 is ONF → high levels of fructose 2,6-bisphosphate → activation of PFK-1 → glycolysis
Pyruvate Kinase Regulaton
final step of glycolysis
inhibitors: phosphorylation by Protein Kinase A; ATP & alanine allosterically; glucagon (hormone-mediated transcription factors)
activators: insulin (via Fructose 2,6-bisphosphate); Fructose-1,6-bisphosphate (allosterically- feedforward stimulation)
Isoezymes
enzymes that perform the same function but are foudn in differen specific tissues
Pyruvate Kinase Deficiency in Erythrocytes
symptoms: hemolytic anemia, abnormal RBC shape, enlarged spleen, elevated bilirubin, & low O2 affinity of hemeglobin
mechanism: RBCs are dependent on glycolysis for ATP, thus deficiency in pyruvate kinase → low ATP → difficult to maintain ion gradients →osmotilitic fragility → hemolytic anemia & abnormal RBC shape
residual enzyme activity of 5-25%
cAMP in liver
increased by: glucagon & epinephrine
effects: glycogenolysis & glyconeogenesis, respectively
cAMP in skeletal muscle
increased by: epinephrine
effect: glycogenolysis & glycolysis
cAMP in adipose
increased by: epinephrine
effect: lipolysis
cAMP in renal tubular epithelium
increased by: antidiuretic hormone (ADH, aka vasopressin)
effect: water reabsorption
cAMP in intestinal muscosa
increased by: vasoactive intestinal polypeptide (VIP), adenosine, & epinephrine
effect: water & electrolyte secretion
cAMP in vascular smooth muscle
increased by: epinephrine on beta-adrenoceptors
effect: relaxation (vasodilation) & growth inhibition
cAMP in broncial smooth muscle
increased by: epinephrine on beta-adrenoreceptors
effect: relaxation (bronchodilation)
cAMP in platelets
increased by: prostacyclin & prostaglandin E
effect: maintance of inactive state
cAMP in adrenal cortex
increased by: ACTH (adrenocorticotropic hormone)
effect: hormone secretion
cAMP in melanocytes
increased by: MSH (melanocyte-stimulating hormone)
effect: melanin synthesis
cAMP in thyroid gland
increased by: TSH (thyroid stimulating hormone)
effect: hormone secretion
Signal Amplification
one message activates more than one effector
homologous desensitization
- occurs in beta-adrenoceptors when epinephrine is bound
- cytolsolic residues are phosphorylated by beta-adrenergic receptor kinase (BARK) or PKA
- phosphorylated residues are binding site for beta-arrestin→ inhibits interaction with Gs-alpha & targets for endocytosis/lysosomal degradation
M2 mAChR
M2 muscarinic acetylcholine receptor
senseitive to muscarine
GPCR regulated ion channel
found in myocardium, presynaptic terminals, ganglionic neurons, CNS neurons, & some smooth muscle
acetylcholine binds receptor→ trimeric G protein activation→ activation of Gi-alpha→ inhibition of adenylyl cyclase→ decrease [cAMP]→ Gbeta/gamma binds & activates K+ channel→ K+ leaves cell→ long hyperpolerization of cell→ decreased HR
cannel closes when Gi-alpha/GDP binds Gbeta/gamma
Rhodopsin
only expressed in membranes of disks in rod cells
7 membrane-spannig domains of opsin covalently linked to light absorbing 11-cis-retinal
photon absorption→ cis to trans-retinal→ conformational change of opsin→ binding & stimulation of trimeric Gt-alpha→ activates PDE (cGMP phosphodiesterase: alpha & beta catalytic subunits & 2 gamma inhibitory subunits) via removal of gamma subunits→ active PDE breaks cGMP into GMP→ decrease [cGMP]→ closing of nucleotide-gated nonselective Na+, K+, & Ca2+→ hyperpolerization (more negative)→ release of neurotransmitters→ brain “I am in the light”
meta-rhodopsin II
aka activated opsin
form of rhodopsin after photon absorption transforms 11-cis-retinal to all-trans-retinal & conformational change of opsin
activated opsin
aka meta-rhodopsin II
form of rhodopsin after photon absorption transforms 11-cis-retinal to all-trans-retinal & conformational change of opsin
light adaptation
rhodopsin kinase phosphorylates opsin: number of phosphorylation sites inversely proportional to ability to activate Gt-alpha→ heavily phosphorylated opsin bond & inhibited by arrestin→ stimulated disassociation of all-trans-retinal→ shut down of rods→ cone system completely takes over
takes minutes
rhodopsin kinase
ser/thr kinase
phosphorylates opsin: number of phosphorylation sites inversely proportional to ability to activate Gt-alpha
dark adaptation
dim light/dark/less photon absorption→ stimulates dephosphorylation of opsin & binding to 11-cis-retinal
20 minutes- several hours
PIP2
phosphatidylinositol 4,5-bisphosphate
cytosolic membrane anchor, allows some proteins to associate with membrane
cleaved by phospholipase C into DAG & IP3
PLC
Phospholipase C
cleaves PIP2 into DAG & IP3
DAG
plasma membrane-associated second messenger
arises from cleavage of PIP2 by PLC
IP3
free cytosolic second messenger
arises from cleavage of PIP2 by PLC
binds IP3-gated Ca2+ channel on ER (four binding sites: one on each identical subunit)→ opens channel→ Ca2+ flows out of ER lumen into cytosol
PLC-beta
protein activated by Gq-alpha via alpha1-adrenoceptor or M3 mAChR
Ca2+ as 2nd messenger
increase [Ca2+]cytosol→ stimulates inactive PKC (protein kinase C) to bind intracellular leaflet→ DAG binds & activates PKC→ autophosphorylation of PKC→ cellular growth & metabolism, regulates glucose metabolism (in liver cells), regulation of transcription factors & gene expression
CaM
calmodulin
- 4 Ca2+ cooperatively bind to activate→ complex modulates many different enzymes
- ex. cAMP & cGMP PDEs
- myosin light chain-kinase
- endothelial nitric oxide synthase (eNOS)
M3 mAChR in blood vessel epithelium
M3 musculinic acetylcholine receptor
acetylcholine binds receptor→ binds & activation of Gq-alpha→ phospholipase C activation→ cleavage of PIP2→ IP3 binds IP3-gated Ca2+ channel in ER (four binding sites: one on each identical subunit)→ opens channel→ Ca2+ flows out of ER lumen into cytosol→ increase [Ca2+]cytosol→ 4 Ca2+ bind to calmodulin→ complex activates eNOS (endothelial nitric oxides synthase→ NO (nitric oxide) synthesis→ NO release→paracrine signaling to vascular smooth muscle, binds & activates sGC (soluble guanylyl cyclase)→ cGMP production→ cGMP binds & activates PKG (protein kinase G)→ inhibition of actin-myosin complex, relaxation of vascular smooth muscle, & vasodilation
RTKs
Receptor Tyrosine Kinases
family of transmembrane proteins with cytosolic domain with tyrosine kinase activity
ligands: many growth factors & insulin
ligand binding→ RTK dimerization→ cross phosphorylation→ phosphotyrosine residues act as binding site for adaptors or docking proteins→ phosphorylation of docked protein
can activate numerous pathways
Ras-MAPK pathway
rat sarcoma-mitogen activated protein kinase pathway
ex. EGF (epidermal growth factor)→ binds EGFR→ EGFR dimerization→ cross phosphorylation→ EGFR phosphotyrosine residues recruit Grb2 (growth factor receptor-bound protein 2)→ recruits & binds sons of sevenless (Sos)→ Sos binds Ras/GDP & assist with dissociation & binding or GTP→ Ras/GTP dissociates from Sos→ stimulates MAPK (Raf)→ phosphorylates MEK→ phosphorylates ERK→ homodiamerization→ phosphorylate & regulate transcription factors c-fos, c-myc, c-jun→ cell growth, proliferation, survival, & progression
PDH complex
pyruvate: passive diffusion through pore of outer mitochondrial membrane & through inner mitochondrial membrane by pyruvate carrier
3 components: E1 (pyruvate dehydrogenase), E2 (dihrdrolipoyl transacetyl), & E3 (dihydrolipoyl dehydrogenase)
needed for carbohydrate oxidation, but not fatty acid oxidation

PDH complex coenzymes
E1 (pyruvate dehydrogenase)→ thiamin
E2 (dihrdrolipoyl transacetyl)→ lipoic acid
E3 (dihydrolipoyl dehydrogenase)→ FAD
Can I Keep Selling Sex For Money, Officer?
citrate→ isocitrate→ alpha-ketoglutarate→ succinyl-CoA→ succinate→ fumarate→ malate→ oxaloacetate
PDH deficiency
lactic acidosis: accumulated pyruvate is converted to lactic acid & alanine
malfunction of brain: b/c it depends on glucose oxidation
severe (neonatal acidosis & death), moderate (brain damage, optical atrophy, mental & motor retardation, & childhood death), & mild (progrssive spinocerebellar ataxia & carbohydrate intolerance)
treated with high fat, low carb diet & dichloroacetate (PDK inhibitor→ PDH is always active)
Pyruvate carboxylase deficiency
decrease in [oxaloacetate]
anaplerotic rxn
chemical reaction that forms intermediates of a metabolic pathway
TCA substrate by tissue type
muscle: uses all (glucose, fatty acids, amino acids, & alsohol) esp. during contraction
liver: uses all after meal, mostly fatty acids during fasting
brain: glucose, ketone bodies during fasting
erythrocytes: anaerobic glycolysis only
Ketone body
made in liver form fatty acids
used for energy in TCA cycle in brain during fasting
PDH Inhibition
allosteric: high ratios of [ATP]/[ADP], [NADH]/[NAD+], & [acetyl-CoA]/[CoA-SH]
phosphorylation: by PDH kinases
PDKs
Pyruvate dehydrogenase kinases
stimulated by: ATP, NADH, Acetyl-CoA, high-fat diet (fat metabolism inhibits carbohydrate metabolism {type 2 diabetes}), fasting, glucocorticoids
inhibited by: ADP, NAD+, CoA-SH, insulin, pyruvate
Arsenite
H2AsO3-
binds to lipoic acid & inactivates it
effect: inhibition of PDH complex→ no acetyl-CoA from pyruvate
Beriberi
thiamin deficiency (from malnutrition)
symptoms: neuromuscular weakness, peripheral vasodilation, heart failure, neuropsychiatric symptoms, carbohydrate intolerance
treatment: thiamin
Wernicke-Korsakoff Syndrome
thiamin deficiency in alcoholics
symptoms: neuropsychiatric symptoms & carbohydrate intolerance
treatment: make sure to give thiamin before glucose
citrate synthase
irreversible, first step of TCA cycle
inhibited by citrate & ATP

aconitase reaction
equilibrium, second rxn of TCA
90% citrate, 7% isocitrate, 3% aconitase
use of isocitrate pulls rxn in proper direction, deltaGº’= 1.6kcal/mol

Isocitrate dehydrogenase
near-irreversible b/c [NAD+] is higher than [NADH] in mitochondria
deltaGº’= -2.0kcal/mol
inhibited by: NADH
stimulated by: ADP & NAD+

alpha-ketoglutarate dehydrogenase
works in a complex like PDH (same subunits & coenzymes), irrevesible
coenzymes: thiamin, lipoic acid, FAD
deltaGº’= -8.0kcal/mol
inhibited by: NADH, ATP, & succinyl-CoA

succinyl thiokinase
aka succinyl-CoA synthetase
substrate-level phosphorylation: GTP in liver & ATP in muscle & brain
deltaGº’= -0.7kcal/mol

succinate dehydrogenase
part of respiratory complex II
not soluble, integral to inner mitochondrial membrane
direct donation of FADH2 electrons to respiratory chain
FAD prostetic group
deltaGº’= 0kcal/mol

fumarase
deltaGº’= -0.9kcal/mol
reversible

malate dehydrogenase
unfavorable equilibrium =, making [oxaloacetate] low in mitochondrion
deltaGº’= 7.1kcal/mol

Products of TCA cycle
2 CO2, 3 NADH, FADH2, 1 GTP or ATP
oxaloacetate
TCA cycle intermediate
also used to make aspartate→ asparagine & pyrimidines
fumarate
TCA cycle intermediate
can also be made from amino acids
citrate
TCA cycle intermediate
used to make fatty acids
alpha-ketoglutarate
TCA cycle intermediate
also used to make glutamate⇔ other amino acids
succinyl-CoA
TCA cycle intermediate
also used to make Heme
can be made from some amino acids
Pyruvate carboxylase
used to maintain or raise [oxaloacetate]

NADH
one reactive site for redox
1:3 ATP
electrons are given up before three proton pumps
FADH2
two reactive redox sites
1:2 ATP made
because it comes into the oxidative phosphorylation pathway after complex I (after one of the proton pumps)
E’º
reduction potential
ability of a substance to participate in redox rxn
Respiratory Chain eletron path
from NADH to complex I or from FADH2 to complex II
from complex I or II to ubiquinone (CoQ10)
to complex II
to cytochrome c
to complex IV to oxygen→water
Coenzyme Q
can accept two electrons, two reduced forms
ubiquinone→ semiquinone→ubiquinol
cytochrome c
small heme protein
carries 1 electron from complex III to complex IV
complex I
NADH-Q Oxidoreductase
where NADH electrons enter respiratory chain
NADH→ FMN⇔FMNH2→ series of 3 iron-sulfur centers→ bound QH2→ series of 4 iron-sulfur centers→ mobile QH2
pumps proton out of mitochondrial matrix into the intermembrane space
NADH + Q + 5H+matrix→ NAD+ + QH2 + 4H+intermembrane
inhbited by: retenone & amytal
Complex II
Succinate-Q Reductase
where FADH2 electrons enter respiratory chain
NOT transmembrane, NOT a proton pump
does contain succinate dehydrogenase enzyme in TCA cycle
electrons transfer from FADH2 to Fe-S centers then to mobile QH2
inhibited by: malonate
complex III
Q-cytochrome c oxidoreductase
transmembrane proton pump from matrix to intermembrane space
moves 1 e- at a time from ubiquinol to cytochrome c
QH2 + 2Cyt cox + 2H+matrix → Q + 2Cyt cred + 4H+intermembrane
blocked by: antimycin A
Complex IV
cytochrome c Oxidase
transmembrane proton pump from matrix to intermembrane space
catalyzes reduction of O2→H2O
electrons: CuA/CuA→ Heme a→ Heme a3/CuB→ Fe3+/Cu2+
4Cyt cred + 8H+matrix + O2→ 4 Cyt cox + 2H2O + 4H+intermembrane
blocked by: CN-, N3-, CO
Chemiosmotic Theory of Peter Mitchell
ATP synthesis comes from the electrochemical gradient across inner mitochondrial membrane created by the energy from breaking down NADH & FADH2
ATP synthase
world’s tiniest rotor powered by proton’s flowing down their electrochemical gradient from intermembrane space into the mitochondrial matrix
c, gamma, & episilon subunits make up the rotor
a, b2, & delta subunits make up stator stationary part
shuttles
required to move material into and out of mitochondrial matrix bc inner membrane is not permeable to most things
glycerol 3-phosphate shuttle
dihydroxyacetone in cytoplasm is reduced by NADH to glyerol 3-phosphate
converted back by an inner mitochondrial membrane protein & reduces FAD to FADH2 that flows down its normal path
found in brown fat
loss of electron→heat

Malate/Aspartate Shuttle
more efficient than glycerol 3-phosphate b/c NADH → NADH, no electron loss

DNP
2,4-dinitrophenol
uncouples ATP synthesis by allowing protons to move back into mitochondrial matrix by alternative pathway
cause weightloss & increase body temp
death by hyperthermia
Regulated Uncoupling
UCP (uncoupling channel protein)
used for generating heat
ROS
Reactive Oxygen Species
supeoxide O2-, hydrogen peroxide H2O2, hydroxyl radical .OH
O2 + e-→ O2- + e-→ H2O2 + e-→ •OH + e-→ H2O
Ground state O2
minimally reactive due to parallel spins of 2 unpaired e-
biradical
paramagnetic
unlikely to participate in rxn with organic molecules
•O-O•
non-reactive oxygen species
Singlet Oxygen
O-O:
non-reactive oxygen species
Superoxide
•O-O:
reactive oxygen species
lasts for milliseconds
perhydroxyl radical
•O-O:H
reactive oxygen species
lasts for seconds
hydrogen peroxide
H:O-O:H
non-reactive oxygen species
lasts for minutes
hydroxyl radical
H:O•
most reactive & thus dangerous ROS
*Fenton Rxn*
lasts for nanoseconds
hydroxyl ion
H:O:
water
H:O:H
Fenton Rxn
H2O2 + Fe2+ (ferrous)→ OH- + •OH + Fe3+ (ferric)
requires the presence of vitamin C (ascorbic acid), unusally acting as a pro-oxidative agent
Fe2+ is extrememly reactive oxidant
oxidative stress
imbalance of the body between making free radicals and quenching them with antioxidants
oxidative damage indicated in cancer, Alzheimer’s, and aging
external triggers: pollution, sunlight, & smoking
Endogenous sources of ROS & NRS
- electron transport in mitochondria- highest amt in cell
- complexes I, II, & II & quinone cycle- usually reduced by cytochrome P450
- cytoplasm: xanthine oxidase, NOS isoforms (NO•)
- ER: microsomal oxidation, CYPs, flavoproteins
- lysosomes: myeloperoxidase
- peroxisomes: oxidases, flavoproteins
- H2O2 production in FA degradation
- transition metals: Fe, Cu
- plasma membrane: lipoxygenases, prostaglandin synthase (PHS), NADPH oxidase
Exogenous sources of ROS
- radiation: sunlight (primarily skin & eyes), x-rays, gamma-rays
- chemicals that form ozone, peroxides, & singlet O2
- chemicals that promote superoxide formation: quinones, aromatics
- chemicals that are metabolised to radicals
- chemicals that release ferritin
lipid peroxidation initiation
LH + X• → L• + XH
lipid peroxidation propagation
L• + O2 → LOO•
LOO• + LH→ L• + LOOH
lipid peroxidation termination
2LOO• → non-radical product
L• + LOO• → non-radical product
L• + L• → non-radical product
some of these non-radical products are still dangerous & reactive
consequences of lipid peroxidation
- structural membrane changes: fluidity, channels, signaling proteins, increase ion permeability
- products for adducts/crosslinks w/non-lipids: protein or DNA
- direct toxicity of product
- DNA damage & mutagenesis
ROS protein targets
cyteine, methionine, tyrosine, histidine, & tryptophan
usually found as 2-oxohistidine, 3-chlorotyrosine, Dopa, & dimers of hydroxylated sromatic amino acids (histidine, tyrosine, & tryptophan)
consequences of protein thiol oxidation
- oxidationof catalytic sites on proteins
- loss of function or abnormal function
- formation of mixed sulfide bonds
- protein protein linkages (RS-SR)
- protein-glutathione linakages (RS-SG)
- alteration of 2º or 3º structure
- increased susceptibility to proteolysis
ROS to DNA
can attack sugar & bases
selective G:C mutations indicative of ROS attack
mitochondrial strand breaks→ abnormal components of electron transport chain→ creates more ROS via electron leakage
consequences of DNA oxidation
- DNA adducts (piece of DNA covalently bonded to chemical)
- AP sites (no base moiety)
- strand breaks
- stimluation of DNA repair
- imbalanced induction of DNA repair enzymes
- induction of error prone polymerases
- can deplete energy reserves
NADPH oxidase
mainly present in neutrophils
NADPH → NADP+ creates •O2-
acetominophen overdose
- metabolized to radicals
- radicals quenched by glutathione
- after 10 days the glutathione runs out & you die
SOD
superoxide dismutase
catalyzes rxn: •O2- + •O2- + 2H+→ H2O2 + O2
CAT
catalase
catalyzes rxn: 2H2O2 → O2 + 2H2O
heme-containing enzyme
important in removal of H2O2 generated by peroxisomes during catabolism of fatty acids & prurine
4 NADPH binding sites/catalase tetramer
NADPH functions to protect against inactivation by H2O2
glutathion
tripeptide: Glu-Cys-Gly
antioxidant capability assisted by sulphydryl group of C
functions as free radical scavenger
stabilizes membrane structure by removing acyl peroxides formed by lipid peroxidation
glutathione peroxidase
catalyzes:
ROOH + 2GSH→ ROH + H2O + GSSG
H2O2 + 2GSH → 2H2O + GSSG
seleno-enzyme
in liver, 2/3 in cytosol, 1/3 in mitochondria
vitamin C
ascorbic acid
directly scavenge oxygen free radicals w/ &w/o enzyme catalysis
water soluble
vitamin E
tocopherol
lipid soluble
free scavenger of lipid peroyx radicals & singlet oxygen
it is reduced by vitamin C
interphase
time when cell is not undergoing mitosis
further divided in G0, G1, S, & G2
G0
Gap 0
quiescent/senescent
resting phase; cell has stopped dividing
occurs when DNA damage or degradation makes cell’s progeny nonvialbe
fully differentiated cells
G1
Gap 1
cell increases in size
G1 checkpoint makes sure cell is ready for DNA synthesis
histone & other DNA replication proteins are made in this phase
S
synthesis phase
DNA replication occurs
G2
Gap 2
cell growth
G2 checkpoint ensures that cell is ready to undergo mitosis
cell makes MT & other proteins for mitosis
phases of wound
injury→ red blood cells & platelets→ coagulation: platelets form fibrin→ early inflammation (24hrs): platelets release TGF-beta & PDGF→ recruits neutrophils→ late inflammation (48hrs): macrophage recruitment→ porliferation (72hrs): collagen & fibroblast formation→ remodeling (weeks to months)
cdks
cyclin-dependent kinases
inactive unless bound to cyclins
active ocomplex phosphorylates downstream targets
cyclins direct cdks to target proteins
cyclins
regulatory proteins that bind and activate cdks
cdk4/cyclin D
rises to G0→ G1 transition
cdk6/cyclin D
rises to G0→ G1 transition
cdk2/cyclin E
rises in G1 & carries through G1→ G2 transition
cdk2/cyclin A
rises in early S & carries thorugh S→ G12 transition
cdk1/cyclin A
rises late S & G2→M transition
MPF
maturation promoting factor
cdk1/cyclin B
G2→M transition
G1 restriction point
check point at end of G1
if passed, cell will usually complete division
cdk2/cyclin E activate transcription factors for genes for DNA synthesis & S phase cdks
STOPPED by: p53→ turning on p21 gene→ p21 protein binds cdk2/cyclinE complex to inhibit it
protein size
kiloDaltons/100= # of aa in protein
Rb
retinoblastoma protein
negative control of cell cycle
active: binds gene regulatory protein & inhibits it
inactivated by: mitogen or growth factor binding→ activates G1-cdk & G1/S-cdk→ phosphorylates (x2) Rb protein→ inactivates Rb→ Rb release of E2F (gene regulatory protein)→ gene transcription
cdc6
cell division cycle 6
cdk2/cyclin A triggers DNA replication via destruction of cdc6
normally bound to origin recognition complex (ORC)
it’s degradation ensures that DNA is only replicated once per cell cycle
CKI
cyclin-dependent kinase inhibitors
control cell cycle progression in response to intra- & extracellular signals
family members: p21, p27, p16
CDC25
phosphatase that dephosphorylates & activates cdks
Wee1
kinase that phosphorylates & inhibits cdks
G2 checkpoint
2nd cell cycle checkpoint, occurs at end of G2
triggers start of mitosis
MPF (cdk1/cyclin B) activates by CDC25
if DNA is damaged than cell cycle is arrested via inactivation of CDC25
Metaphase checkpoint
bipolar tension on chromosomes aligned at mitotic plate
tension is sensed→ degradation of cyclin B (which harbors D-box (destruction box)→ allows anaphase promoting complex (APC) to form→ APC breaksdown securin (inhibits separase)→ separase hydrolyzes cohesin→ separation of sister chromatids
cdk1 regulation
- phosphorylated & inactivated by Wee1
- APC-mediated ubiquination of cyclin B
- CDC25 phosphatase activate of p-cdk-cyclin via removal of phosphoryl group
Separase
protein responsible for cohesin breakdown & sister chromatid separation
securin binds→ separase folds→ separase/securin complex phosphorylated by cdk1/cyclin B→ APC destroys securin→ CDC20 dephosphorylates separase→ separase is active→ cleaves cohesin
allele
different versions of a gene
gene
transcription unit or allele, depends on context
genotype
individual’s collection of genes or refers to two alleles of a given gene
heterozygote
individual with two different alleles at a gene
homozygote
individual with two of the same alleles at a given gene
hemizygote
has only one allele for a given gene
should only occur for sex chromosomes in males
haploinsufficiency
one allele for a gene in which they should have two alleles
phenotype
characteristic of a person
expression pattern for a gene + environment
gamete
either egg or sperm
affected
person with symptoms
congenital
symptoms present at birth (regardless of detection)
might be genetic or due to in utero infection
dominant dz
homozygous dominant & heterozygous will be affected
homozygous dominant very rare & usually occur as new mutation
most common: familial hypercholesterolemia
rare dzs
fitness
number of offspring that reach reproductive age/average number for the population
dominant dz: reduced fitness→ expect lower incidence in next generation
if constant, then assume new mutation
recessive dz
only homozygous recessive affected
normally offspring of heterozygotes
new mutations are RARE
proband
person or patient that brought gene/allele/dz to attention of person making the pedigree
autosomal inheritance recognition
recessive: unaffected parents, 1/4 affected sibship, parents distantly related
dominant: all have affected parent (every generation), 50% affected sibship, new mutation EXCEPTION
autosomal b/c equal # male/female
sex-linked inheritance recognition
x-linked recessive: more males than females b/c hemizygous, never father to son, NEW mutations
x-linked dominant: always show phenotype, more females than males, never father to son
y-linked: male fertility & non-syndromic hearing impairment, only father to son
x-linked recessive transmission
if affected mother, 50% sons affected & 50% daughters carriers
if affected father, all daughters carriers, no sons affected/carriers
more affected males than females
x-linked dominant transmission
more affected females than males, no such thing as carriers
if mother affected, 50% sons affected & 50% daughters affected
if father affected, all daughters affected, no sons affected/carrier
sex-limited inheritance
autosomal gene, dominant or recessive
influences traits expressed in one sex only or trait is influenced by sex hormone
ex. ovarian cancer, testicular cancer, male patterned baldness, autosomal infertility
reduced penetrance
characteristic of a dz
non-penetrant is someone with dz genotype, but no symptoms
can be seen in dominant & receessive dz
variable expressivity
differences in severity or age of onset for an “identical” genotype
mitochondrial inheritance
female carrier gives to all of her offspring
1:10,000 from father
locus heterogeneity
similar or identical phenotype caused by involvment of different genes (@ different loci)
allelic heterogeneity
similar or identical phenotype caused by different alleles at the same locus (gene)
can also mean different alleles at same locus produce different dz
phenocopy
looks like a known genetic disorder, but caused by environmental influence
pleiotropy
mutation in one gene has more than one effect, in more than one physiological system
contiguous gene disorders
typically in deletion or duplication of part of a chromosome
loci are next to each other
Barr body
2nd X chromosome in females
dormant, but chosen randomly per cell so different in different cells
easily detected in buccal swab, but more accurate in karyotype
lyonization
randomaly chosen X inactivation for each cell in females
irreversible for that cell & its descendants
X-heterozygote female displays a mosaic expression of that trait
mosaic
anyone with two types of cells that differ more form each other than they would in most other people
p arm
shorter upper arm of chromosome
q arm
longer bottom arm of chromosome
karyotyped cells
PBMCs in metaphase
steps to karyotyping
- collect blood sample
- add phytohaemagglutinin
- culture for 3 days at 37º
- add colchicine (to block cells in metaphase) (& ethanol or methanol for fixation) & hypotonic saline (w/o this teh chromosomes are too densly packed)
- slide
- digest w/trypsin & stain w/Giemsa
- analyze spread
cholchicine
spindle apparatus inhibitor that arrests cells in metaphase
phytohemagglutinin
lectin that induces white blood cells to divide
metacentric chromosome
p & q arms of equal length
submetacentric chromosomes
when p arms are shorter than q arms
acrocentric chromosomes
when p arms are almost nonexistant, referred to as satellites
group D chromosomes
larger acrocentric chromosomes (13-15)
group G chromosomes
small acrocentric chromosomes 21 & 22
chromosome bands
darkened bands contain fewer transcriptionally active genes therefore have more condense chromatin structure & high in A-T base pairs
polyploidy
3 or 4 copies of all chromosomes
most common reason for early pregnancy loss
trisomy
one extra chromosome
only viable if 13, 18, 21, X, or Y
16 is the most common (but NOT viable)
caused by nondisjunction in meiosis I
Monosomy
missing a chromosome
only viable if X or Y, must still have one X
caused by nondisjunction in meiosis I
chromosomal deletion
part chromosome is deleted or lost
unbalanced karyotype
chromosomal inversion
part of a chromosome is cut out & flipped around & reinserted
balanced mutation
duplication
segment is in proper place & in another place (sometimes right next to each other)
unbalanced, excess mutation
insertion
a segment has cut & reinserted elsewhere
balanced, but could be unbalanced in offspring
ring chromosome
telomeres have been deleted & chromosome forms a ring to protect DNA
isochromosome
chromosome with identical arms (p=q)
stable reciprocal translocation
occurs when there is an exchange of two acentric (no centromere) fragments
results: multiple affected systems→ syndromes, most commonly: mental retardation & heart defects
unstable reciprocal translocation
occurs when there is an exchange of an acentric (no centromere) fragment with a centric fragment
resulting in a dicentric & acentric chromosome
Robertsonian translocation
when acrocentric chromosomes translocate p-p & q-q
often p-p chromosome is lost, but doesn’t affect viability
acrocentric chromosomes: 13-15, 21, & 22
Klinefelter syndrome
47, XXY
1/2 diagnosed as school aged boys for developmental delay or learning disability & social maladjustment
other 1/2 when seeking help for infertility
long limbs, small testis→reduced levels of testosterone→ high pituitary gonadotropins, reduced sexual function, & gynecomastia (55%)
55% from mother, 45% from father
also seen: obesity, diabetes, thryroid problems, & pulmonary dz
SRY
master control gene on Y chromosome
intersex
presence of both ovarian & testicular tissue at birth
variable phenotype
most karyotype as 46XX some with SRY
female intersex
have ovaries but virilized phenotype
causes: prenatal exposure to progesterone or androgen OR inherited deficiency of 11- or 21-hydroxylase in adrenal cortex
adrenogenital syndrome
ambiguous external appearance
hyperplasia (increased size due to increased cellular reproduction) of adrenal cortex
2/3 have hyponatremia & hyperkalemia
treated w/cortisol
11- or 21-hydroxylase deficiency
autosomal recessive inheritance
11: 5% of cases, normal or hypokalemia, 2/3 w/HTN
21: 95% of cases, hyponatremia & hyperkalemia, mild form displays hirutism & advanced bone age
androgen insenitivity syndrome
externally & psychosexually female, no uterus or Fallopian tubes, no Wolffian ducts, non-descended testes in abdomen
x-linked recessive
presents as primary amenorrhea or inferility
testes should be removed- prone to malignancies
5alpha-reductase type 2 deficiency
46, XY
partial feminization at birth, Wolffian structures present, but testes often non-descended, any range of external genitalia
autosomal recessive
no conversion of testosterone to dihydrotestosterone
usually raised as girls, but identify as male

