Structures - Medical Chemistry Flashcards
(139 cards)
1
Q
a-ketoglutaric acid
A

2
Q
aluminium chloride
A
AlCl3
2
Q
acridine
A

2
Q
acetaldehyde
A

2
Q
acetone
A

2
Q
acetic acid
A

2
Q
acetoacetic acid
A

3
Q
bromous acid
A
HBrO2
3
Q
ammonium sulfate
A
(NH4)2SO4
3
Q
ammonia
A
NH3
3
Q
boric acid
A
H3BO3
3
Q
benzene
A

3
Q
butanol
A
CH3-CH2-CH2-CH2-OH
3
Q
aniline
A

3
Q
butyric acid
A
CH3-CH2-CH2-COOH
3
Q
anhydride
A

3
Q
amide
A

4
Q
calcium chloride
A
CaCl2
6
Q
calcium sulfate
A
CaSO4
7
Q
caproic acid
A
CH3-CH2-CH2-CH2-CH2-COOH
8
Q
carbon monoxide
A
CO
8
Q
carbon dioxide
A
CO2
9
Q
citric acid
A

9
Q
cis-aconitic acid
A

9
common names of C1 - C6 carboxylic acids
formic (C1) -\> acetic (C2) -\> proprionic (C3) -\>
butyric (C4) -\> valeric (C5) -\> caproic (C6)
FAP BVC
10
copper sulfate
CuSO4
11
diethylether
H3C-CH2-O-CH2-CH3
11
common names of C2 - C5 dicarboxylic acids
oxalic (C2) -\> malonic (C3) -\>
succinic (C4) -\> glutaric (C5)
OMSG
12
dipotassium hydrogen phosphate
K2HPO4
12
ester

14
ethanol
CH3-CH2-OH
14
ether

15
ethylene
H2C=CH2
17
ferrous ammonium sulfate
Fe(NH4)2(SO4)2
18
fluoroapatite
Ca5(PO4)3F
19
formaldehyde

21
formic acid

22
fumaric acid

23
furane

24
glycerol

24
glutaric acid

25
glycol
HO-CH2-CH2-OH
26
guanidine

27
hemiacetale

28
hydrazine
H2N-NH2
28
hemiketale (cyclic forms included)

28
hydrazone

30
hydrochloric acid
HCl
31
hydrogen cyanide
HCN
31
hydrogen peroxide
H2O2
33
hydrogen sulfide
H2S
35
hydroxamic acid

37
hydroxyapatite
Ca5(PO4)3OH
39
hydroxylamine
HO-NH2
40
hypochlorous acid
HClO
41
imidazole

42
indole

43
inositol

43
Explain the rules of the **-ous/-ic system**
* lower oxidation number uses the suffix "-**ous**"
* higher oxidation numbers uses the suffix "**-ic**"
_Oxidation numbers:_
* ferrous/ferric = Fe2+/Fe3+
* cuprous/cupric = Cu+/Cu2+
* mercurous/mercuric = Hg+/Hg2+
44
isocitric acid

45
isoprene (2-methyl-1,3-butadiene)
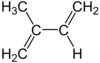
46
lactic acid

47
lactone

48
magnesium sulfate
MgSO4
50
maleic acid

51
malic acid

52
malonic acid

53
manganese chloride
MnCl2
54
mercaptoethanol
HO-CH2-CH2-SH
55
mercurous chloride (calomel)
Hg2Cl2
57
mercury chloride
HgCl2
58
metaphosphoric acid
(HPO3)n
59
methanol
CH3-OH
60
naphtalene

62
nitric acid
HNO3
63
nitric oxide
NO
64
nitrous acid
HNO2
66
nitrous oxide
N2O
67
orthophosphoric acid
H3PO4
68
oxalic acid
HOOC-COOH
70
oxaloacetic acid

71
oxazole

72
oxime

74
perchloric acid
HClO4
75
phenantrene

77
phenol

79
potassium biiodate
KH(IO3)2
81
potassium cyanide
KCN
82
potassium ferricyanide
K3[Fe(CN)6]
84
potassium hydrogencarbonate
KHCO3
85
potassium iodide
KI
86
potassium nitrate
KNO3
88
potassium permanganate
KMnO4
89
potassium sulfite
K2SO3
90
propanol
CH3-CH2-CH2-OH
91
proprionic acid
CH3-CH2-COOH
92
pteridine

93
purine

94
pyrane

96
pyrazine

97
pyrazole

98
pyridine

99
pyrimidine

100
pyrrole

102
pyruvic acid

104
Schiff-base

105
silver chloride
AgCl
106
silver chromate
Ag2CrO4
107
silver nitrate
AgNO3
108
sodium carbonate
Na2CO3
109
sodium chlorate
NaClO3
110
sodium chloride
NaCl
111
sodium chlorite
NaClO2
113
sodium dihydrogen phosphate
NaH2PO4
114
sodium hydroxide
NaOH
115
sodium hypochlorite
NaClO
116
sodium nitrite
NaNO2
117
sodium periodate
NaIO4
118
sodium pyrophosphate
Na4P2O7
119
sodium thiosulfate
Na2S203
120
succinic acid

122
sulfinic acid
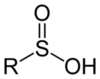
123
sulfonic acid

124
sulfoxide

125
sulfur dioxide
SO2
126
sulfur trioxide
SO3
127
sulfuric acid
H2SO4
128
sulfurous acid
H2SO3
129
superoxide anion
O2-
130
thiazole

131
thioester

132
thioether

133
thiol

134
thiophene

135
urea

136
valeric acid
CH3-CH2-CH2-CH2-COOH
137
water
H2O
138
zinc chloride
ZnCl2
139
ß-hydroxybutyric acid



