TOPIC 17 - Organic chemistry II Flashcards
(50 cards)
What are optical isomers?
Mirror images of each other that have a chiral carbon.
What is a chiral molecule?
Molecule that has 4 different groups attached to a carbon atom. These groups can be arranged in two different ways (enantiomers).
What are enantiomers?
Isomers that are non-superimposable images of each other.
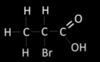
Draw the two enantiomers of this molecule

What is plane polarised light?
Light that only oscillates in one plane.
One enantiomer rotates plane polarised light 10º clockwise. What effect does the other enantiomer have?
Rotates plane polarised light 10º anticlockwise.
What are racemates?
Racemic mixtures, which are mixtures that are made from a equal amount of enantiomer.
Do racemates rotate plane polarised light?
No. The 2 enantiomers have opposite effects and so cancel out.
How can SN1 reactions produce racemic mixtures?

How can SN2 reactions produce only 1 enantiomer?

What is the boiling point for aldehydes + ketones in comarison to alcohols?
Lower bpts than alcohols.
Can aldehydes+ketones hydrogen bond with water?
Yes
Can aldehydes+ketones dissolve in water? Why?
Yes. But only the small ones since with bigger ones, London forces between the hydrocarbon chains become stronger than the H-bonding with water and so they don’t dissolve.
How can we distinguish between aldehydes and ketones using K2Cr2O7 ?
Aldehydes are oxidised by K2Cr2O7, while ketones aren’t.
If aldehyde is present solution turns from orange (Cr2O7 2-) to green (Cr3+). If ketone is present, there’s no change.
How can we use Tollen’s reagent to distinguish between aldehydes and ketones?
- Place aldehyde/ketone in hot water bath with Tollen’s reagent.
- No bunsen burner because aldehydes/ketones are flammable.
- If aldehydes are present, a silver coating is seen.
- If ketones present, no change.
How is Tollen’s reagent made?
Reacting silver nitrate with aqueous ammonia.
What is the general equation for the reaction of Tollen’s reagent with an aldehyde?

How can we use Fehling’s or Benedict’s slution to distinguish between aldehydes and ketones?
Add both solutions to warm water bath.
If aldehyde present, solution turns from blue to a brick red precipitate (Cu2O).
If ketones are present, there’s no change.
What is the difference between Fehling’s and Benedict’s solution?

What is the equation for the reaction of Fehling’s / Benedict’s solution with aldehyde?

What are the conditions for the reduction of aldehydes and ketones into alcohols?
NaBH4 dissolved in methanol and water.
What is the mechanism for the reaction between carbonyl compounds and potassium cyanide?

What type of mechanism is the reaction between carbonyl compounds and potassium cyanide to form hydroxynitriles?
Nucleophilic addition.
Can hydrogen cyanide (instead of potassium cyanide) react with a carbonyl group to give a hydroxynitrile?
Yes. But no acid is needed.